Surprising results from the heaviest element ever chemically studied
Discover how groundbreaking research on superheavy elements is reshaping our understanding of the periodic table and paving the way for future innovations.
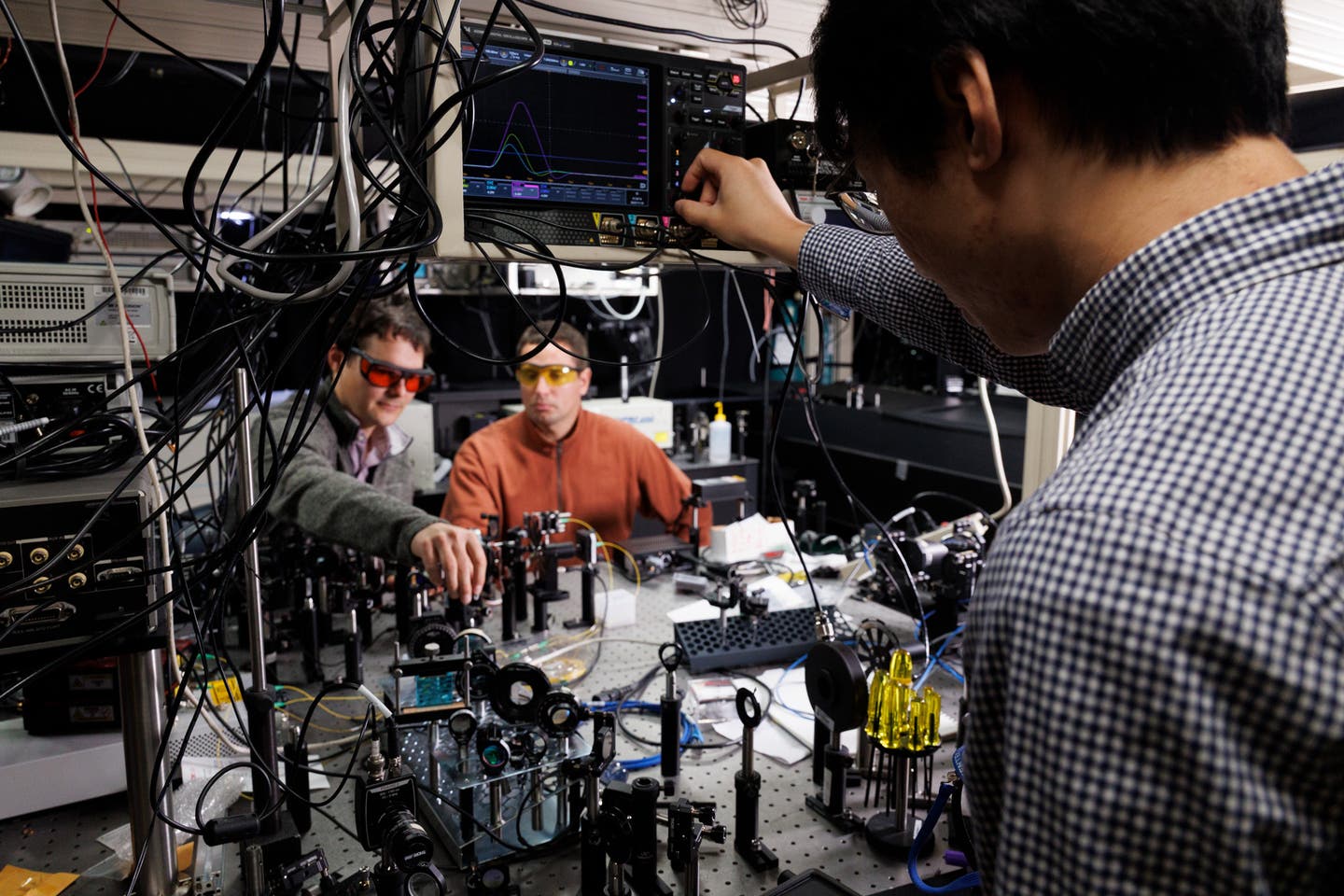
Dr. Alexander Yakushev, spokesperson of the experiment (right) and Dominik Dietzel, PhD student from Johannes Gutenberg Mainz University, work on the detector channel used to register the short-lived nihonium and moscovium atoms. (CREDIT: A. Yakushev)
In recent decades, researchers have pushed the boundaries of the periodic table, synthesizing the heaviest elements known to science. These superheavy elements (SHE) complete the seventh row of the periodic table, culminating in element 118, oganesson.
However, creating these elements is no small feat. Production rates plummet as atomic numbers rise, with only one atom of oganesson produced per month. Such scarcity makes studying the chemical properties of these fleeting elements an extraordinary challenge.
To isolate these elements, scientists rely on advanced techniques like kinematic separation in recoil separators. Within microseconds, superheavy isotopes are captured, enabling studies of their nuclear decay. Yet chemical studies are far more demanding, particularly for isotopes with half-lives measured in mere seconds.
Fast, efficient methods like gas-solid chromatography help researchers examine volatile species of these elusive elements.
Understanding the Role of Relativity in Superheavy Elements
For elements this heavy, relativistic effects significantly influence chemical behavior. As atomic numbers increase, electrons orbit their nuclei at relativistic speeds, altering their mass and energy levels. This phenomenon affects the reactivity and bonding properties of superheavy elements.
Related Stories
Notably, the contraction of s and p1/2 atomic orbitals, coupled with large spin-orbit splitting, sets superheavy elements apart from their lighter counterparts. For instance, elements like copernicium (Cn) and flerovium (Fl) exhibit unusually low reactivity, a characteristic supported by experimental studies.
"These predictions were recently confirmed experimentally," notes Schwerdtfeger and colleagues, solidifying the understanding of SHE chemistry.
Moscovium and Nihonium: Shedding Light on New Frontiers
Recent studies led by GSI Helmholtzzentrum für Schwerionenforschung in Darmstadt have brought moscovium (element 115) and nihonium (element 113) into the spotlight. These elements, chemically more reactive than flerovium, represent a new frontier in superheavy chemistry.
The experiments mark moscovium as the heaviest element ever chemically studied, a milestone published in Frontiers in Chemistry.
This research fills critical gaps in the periodic table, offering new insights into the structure and behavior of these elements. As Dr. Alexander Yakushev explains, "Thanks to a newly developed setup for chemical separation and detection... we were able to observe the very short-lived moscovium-288."
How the Experiments Work
Producing superheavy elements requires intricate techniques. Researchers bombarded americium-243 targets with calcium-48 ions, creating moscovium-288. This isotope decays within milliseconds into nihonium-284. Inert gas carries these elements through a detector array, coated in quartz, to observe their interactions.
The team measured chemical reactivity by observing how strongly atoms bonded with the quartz surface. Less reactive atoms continued through to gold-covered detectors. Gold, known for stronger bonds, ensured accurate data capture. Over two months, the team observed four moscovium and 14 nihonium atoms.
The results were striking. While moscovium and nihonium bonded weakly with quartz, suggesting moderate reactivity, their lighter homologs—bismuth and thallium—formed strong bonds. This behavior highlights the unique relativistic effects at play, particularly in superheavy elements like flerovium, which barely reacts, mimicking noble gas behavior.
Implications for the Periodic Table
These findings showcase the profound influence of relativistic physics on the periodic table's heaviest members. Flerovium's near-inertness stems from closed electron subshells, reminiscent of noble gases. This challenges traditional periodic trends and underscores the limits of extrapolation from lighter elements.
As elements like nihonium and moscovium behave differently from expected, scientists refine their understanding of periodicity. Such insights are crucial for future applications, though practical uses for these elements remain speculative.
Future Prospects: Beyond Chemistry
While industrial applications of superheavy elements like flerovium remain distant, the knowledge gained could pave the way for future breakthroughs. Advancing synthesis techniques may eventually yield stable quantities, potentially revolutionizing materials science, energy storage, or even medicine.
The ongoing construction of the Facility for Antiproton and Ion Research (FAIR) in Darmstadt promises to expand these investigations. As technology evolves, so too will our capacity to harness the unique properties of the heaviest elements.
By decoding the chemical character of moscovium and nihonium, researchers lay a foundation for exploring uncharted realms of the periodic table. The work at GSI/FAIR exemplifies human ingenuity, pushing the boundaries of knowledge and shaping the future of scientific discovery.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.