Study reveals potential eye risk for Semaglutide (Ozempic) users
Study has found a troubling link between the use of semaglutide (marketed as Ozempic or Wegovy) and an increased risk of developing a potentially blinding eye condition
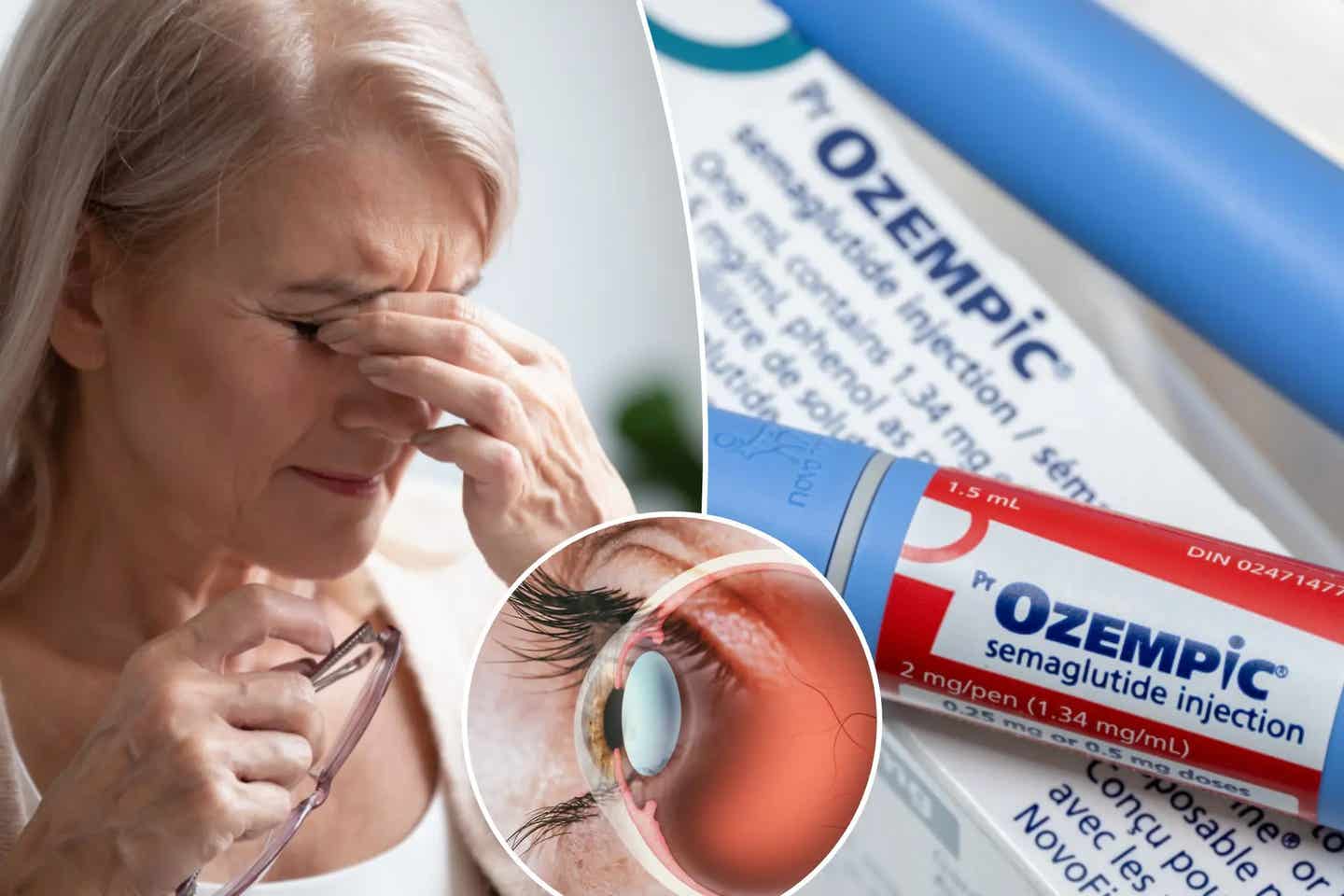
Study has found a troubling link between the use of semaglutide (marketed as Ozempic or Wegovy) and an increased risk of developing a potentially blinding eye condition. (CREDIT: Creative Commons)
A recent study led by researchers at Mass Eye and Ear has found a troubling link between the use of semaglutide (marketed as Ozempic or Wegovy) and an increased risk of developing a potentially blinding eye condition known as NAION (non-arteritic anterior ischemic optic neuropathy). This risk was significantly higher in patients prescribed semaglutide for diabetes or weight loss compared to those who were not.
Specifically, the study revealed that individuals with diabetes who were prescribed and filled a prescription for semaglutide were more than four times more likely to be diagnosed with NAION. Similarly, those prescribed the drug for obesity or overweight were over seven times more likely to receive the same diagnosis.
The study, led by Dr. Joseph Rizzo, director of the Neuro-Ophthalmology Service at Mass Eye and Ear and the Simmons Lessell Professor of Ophthalmology at Harvard Medical School, was published in JAMA Ophthalmology.
"The use of these drugs has exploded throughout industrialized countries and they have provided very significant benefits in many ways, but future discussions between a patient and their physician should include NAION as a potential risk," stated Rizzo. “It is important to appreciate, however, that the increased risk relates to a disorder that is relatively uncommon.”
NAION is rare, affecting up to 10 out of 100,000 people in the general population. It is the second-leading cause of optic nerve blindness, following glaucoma, and the most common cause of sudden optic nerve blindness. NAION is believed to result from reduced blood flow to the optic nerve head, leading to permanent visual loss in one eye. According to Rizzo, the vision loss caused by NAION is painless, progresses over several days, and has little potential for improvement. Currently, there are no effective treatments for NAION.
The study was prompted in late summer 2023 when Rizzo and his colleagues, including study co-author Dr. Seyedeh Maryam Zekavat, noticed an unusual trend. Three patients in their practice had been diagnosed with vision loss from NAION within a single week, and all three were taking semaglutide.
This observation led the research team to conduct a retrospective analysis of their patient population to explore a potential link between semaglutide and NAION. Semaglutide, initially developed for treating type 2 diabetes, has gained popularity for its weight loss benefits since its release as Ozempic in 2017 and as Wegovy in 2021 for weight management.
The researchers reviewed the records of over 17,000 Mass Eye and Ear patients treated since Ozempic's release. They divided these patients into those diagnosed with diabetes or overweight/obesity and compared those who had received prescriptions for semaglutide with those taking other diabetes or weight loss drugs. The analysis revealed a significant increase in the rate of NAION diagnoses among those prescribed semaglutide.
However, the study had several limitations. Mass Eye and Ear treats an unusually high number of patients with rare eye diseases, the study population was predominantly white, and the number of NAION cases over the six-year study period was relatively small. Small case numbers mean that statistics can shift quickly, as noted by Rizzo. Additionally, the researchers could not determine whether patients actually took their medication consistently or if they started and then stopped taking semaglutide, which could have impacted their risk.
Importantly, the study does not establish causality, and the researchers do not yet understand why this association exists or why the risk differences were observed between diabetic and overweight groups.
"Our findings should be viewed as being significant but tentative, as future studies are needed to examine these questions in a much larger and more diverse population,” said Rizzo. “This is information we did not have before and it should be included in discussions between patients and their doctors, especially if patients have other known optic nerve problems like glaucoma or if there is preexisting significant visual loss from other causes."
This study underscores the need for cautious use of semaglutide, particularly among patients at risk of eye conditions, and highlights the importance of ongoing research to fully understand the drug's implications. As the use of semaglutide continues to grow, so too does the need for comprehensive patient-physician discussions about its potential risks and benefits.
Note: Materials provided above by the The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.
Joseph Shavit
Head Science News Writer | Communicating Innovation & Discovery
Based in Los Angeles, Joseph Shavit is an accomplished science journalist, head science news writer and co-founder at The Brighter Side of News, where he translates cutting-edge discoveries into compelling stories for a broad audience. With a strong background spanning science, business, product management, media leadership, and entrepreneurship, Joseph brings a unique perspective to science communication. His expertise allows him to uncover the intersection of technological advancements and market potential, shedding light on how groundbreaking research evolves into transformative products and industries.