Scientists recreate mouse from reprogrammed stem cells
Researchers use ancient genes from single-celled organisms to create mouse stem cells, uncovering a billion-year evolutionary link.
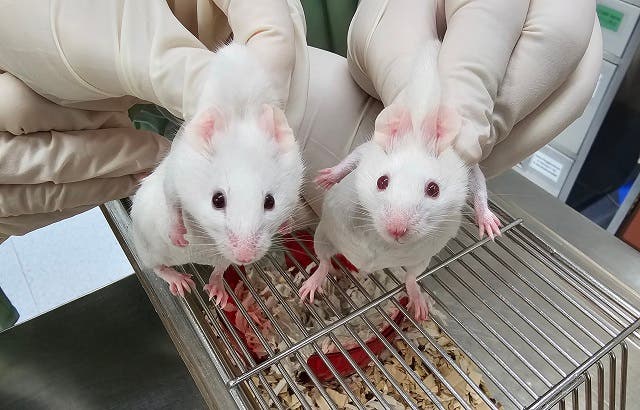
The mouse on the left is a chimeric with dark eyes and patches of black fur, a result of stem cells derived from a choanoflagellate Sox gene. The wildtype mouse on the right has red eyes and all white fur. The colour difference is due to genetic markers used to distinguish the stem cells, not a direct effect of the gene itself. (CREDIT: Gao Ya and Alvin Kin Shing Lee)
The emergence of multicellular life roughly 700 million years ago was a pivotal event that shaped the evolution of life on Earth.
Key to this transformation was the development of stem cells, which possess the unique ability to either replicate themselves or differentiate into specialized cell types. These pluripotent stem cells play a foundational role in forming the complex structures and functions of multicellular organisms, including humans.
Stem cells fall into categories based on their potential. Pluripotent stem cells can become any cell type within the body, while multipotent stem cells are more limited, specializing in certain lineages. For instance, neural stem cells differentiate into neurons, astrocytes, and oligodendrocytes.
In vertebrates, pluripotent stem cells only exist during early embryonic stages, driven by specific transcription factors (TFs) like Sox2 and Oct4. These "pioneer" TFs are crucial, binding tightly to DNA even when it is wrapped in nucleosomes, and directing gene activity necessary for stem cell function.
Sox2 and Oct4 are central players in maintaining pluripotency. Without them, stem cells lose their capacity for self-renewal and differentiation. This partnership is essential during early embryonic development and in laboratory settings, such as in the generation of induced pluripotent stem cells (iPSCs).
These cells, created by reprogramming differentiated cells, rely on a cocktail of factors, including Sox2 and Oct4, to regain their pluripotent state.
Research has long held that Sox and POU family genes, like Oct4, emerged uniquely within the animal kingdom. However, a groundbreaking study published in Nature Communications challenges this view, suggesting these genes predate multicellular animals.
Dr. Alex de Mendoza of Queen Mary University of London, along with colleagues from The University of Hong Kong, achieved a scientific milestone by using genetic tools from choanoflagellates—unicellular relatives of animals—to reprogram mouse cells into pluripotent stem cells.
Related Stories
Choanoflagellates, single-celled organisms that share a common ancestor with animals, possess versions of Sox and POU genes. These genes were long thought to be exclusive to animals, yet their functionality in creating pluripotent stem cells demonstrates an ancient evolutionary link.
“By successfully creating a mouse using molecular tools derived from our single-celled relatives, we’re witnessing an extraordinary continuity of function across nearly a billion years of evolution,” said Dr. de Mendoza.
In a series of experiments, the research team introduced choanoflagellate Sox genes into mouse cells, replacing the native Sox2 gene. The reprogrammed cells were then injected into a developing mouse embryo.
The resulting chimeric mice displayed physical traits from both the donor embryo and the reprogrammed stem cells, such as black fur patches and dark eyes. This confirmed that ancient Sox genes were capable of functioning in a complex multicellular organism.
The implications of this study stretch beyond evolutionary biology. By tracing the ancient origins of stem cell machinery, researchers gain insights that could enhance regenerative medicine. Understanding how these genes evolved and functioned in early life forms might lead to innovations in cell reprogramming and therapy development.
Dr. Ralf Jauch, a co-author of the study, emphasized the potential for future applications. “Studying the ancient roots of these genetic tools lets us innovate with a clearer view of how pluripotency mechanisms can be tweaked or optimized,” he said.
Synthetic versions of these ancient genes could potentially outperform native animal genes in specific therapeutic contexts, opening new avenues for medical advancement.
This research underscores the evolutionary adaptability of genetic tools. While choanoflagellates do not possess stem cells, they use Sox and POU genes for basic cellular functions. Over time, these genes were repurposed in multicellular animals to support stem cell formation and specialization.
“Choanoflagellates don’t have stem cells,” explained Dr. de Mendoza, “but they have these genes, likely to control basic cellular processes that multicellular animals probably later repurposed for building complex bodies.”
The findings highlight how evolutionary recycling allows organisms to innovate using pre-existing molecular tools. By examining the genetic building blocks from our distant unicellular ancestors, scientists can better understand the development of complex life and harness this knowledge to advance human health.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Rebecca Shavit
Science & Technology Journalist | Innovation Storyteller
Based in Los Angeles, Rebecca Shavit is a dedicated science and technology journalist who writes for The Brighter Side of News, an online publication committed to highlighting positive and transformative stories from around the world. With a passion for uncovering groundbreaking discoveries and innovations, she brings to light the scientific advancements shaping a better future. Her reporting spans a wide range of topics, from cutting-edge medical breakthroughs and artificial intelligence to green technology and space exploration. With a keen ability to translate complex concepts into engaging and accessible stories, she makes science and innovation relatable to a broad audience.



