Scientists discover why lungs are so prone to developing cancer
Aspartate boosts lung metastases by triggering eIF5A hypusination, driving aggressive cancer growth. New insights offer hope for targeted therapies.
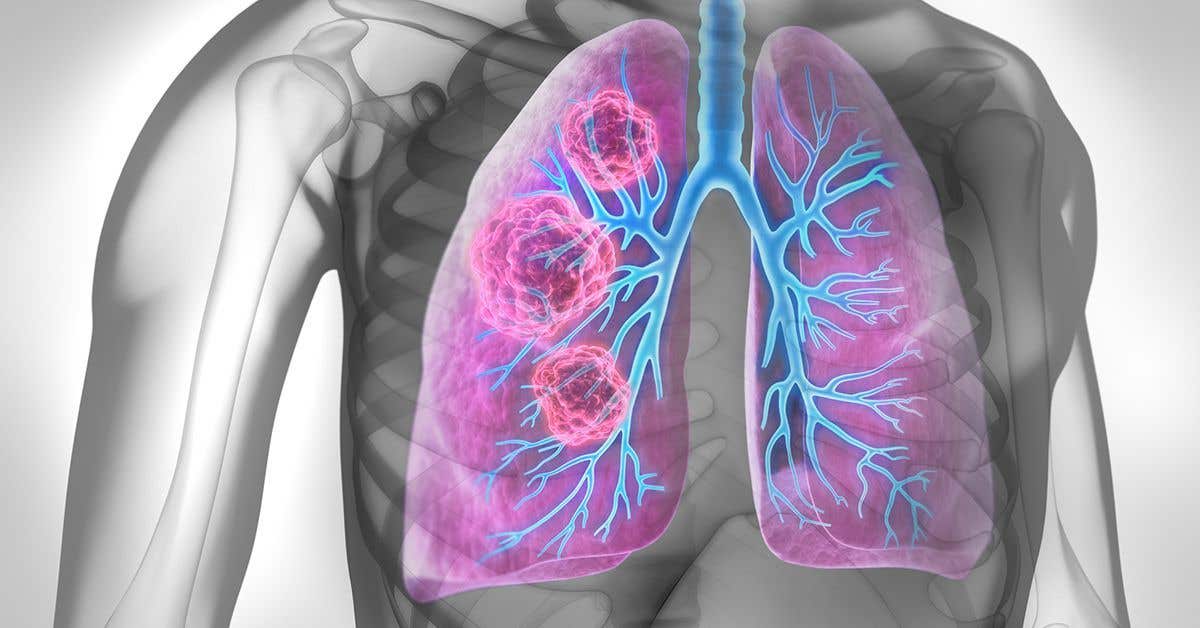
Discover how aspartate amplifies lung metastases by activating cellular pathways in cancer cells. Innovative research points to new treatment possibilities. (CREDIT: Nature)
Lung metastases are a devastating reality for over half of cancer patients whose tumors spread beyond their initial sites. These secondary lung tumors develop in part because of unique conditions within the lungs.
Researchers have been striving to unravel the mechanisms that drive their aggressiveness, and recent findings spotlight the amino acid aspartate as a key player.
The lungs’ structure and biochemistry make them a prime site for metastasis. Their extensive vascular network facilitates the trapping of circulating cancer cells. Additionally, their less oxidative environment offers a safer haven for these cells.
However, another critical factor lies in the lungs’ ability to create a pre-metastatic niche—a welcoming environment shaped by tumor-secreted factors (TSFs) from primary tumors.
Tumor-secreted factors alter lung immune cells and extracellular matrix, priming the site for incoming cancer cells. They also increase the availability of specific nutrients in the lung interstitial fluid, which directly affects cancer cell behavior. One nutrient, aspartate, emerged as a central player in this process.
Aspartate’s unique role in this dynamic has intrigued scientists. Unlike other nutrients, which often require direct uptake by cells, aspartate’s function relies on its ability to act as a signaling molecule. This discovery challenges traditional views on how nutrients influence cancer progression.
Aspartate, typically scarce in blood plasma, was found in surprisingly high concentrations within the lung interstitial fluid of mice and patients with metastatic breast cancer. This elevated level was not observed in mice with non-metastatic primary breast tumors or in other organs, suggesting a unique association between aspartate and lung metastases.
To investigate further, researchers pre-treated mice with aspartate before introducing breast cancer cells. Published in the journal, Nature, this pre-treatment mirrored the high aspartate levels observed in metastatic lungs. The result? Increased aggressiveness of lung metastases and a rise in the activity of a protein called eIF5A, which plays a critical role in protein synthesis.
“We found high levels of aspartate in the lungs of mice and patients with breast cancer compared to those without cancer,” explains Ginevra Doglioni, lead author of the study. “This suggests that aspartate may be crucial for lung metastasis.”
Related Stories
Aspartate’s effects on metastases involve a complex signaling cascade. While most nutrients enter cancer cells through transport mechanisms, aspartate acts differently. It binds to NMDA receptors on the surface of cancer cells.
This interaction triggers intracellular signaling pathways that promote the expression of deoxyhypusine hydroxylase (DOHH), an enzyme essential for modifying eIF5A through a process called hypusination.
Hypusinated eIF5A, in turn, activates a translational program that enhances the ability of cancer cells to thrive in the lung environment. This program includes increased collagen synthesis and TGFβ signaling, which remodels the extracellular matrix to support aggressive tumor growth.
This discovery highlights how cancer cells exploit existing biochemical pathways to adapt and flourish in new environments. Aspartate’s role as a signaling molecule rather than a mere nutrient represents a paradigm shift in understanding metastatic progression.
The findings were validated by examining lung tumor samples from patients with metastatic breast cancer. These samples revealed elevated levels of hypusinated eIF5A and increased expression of NMDA receptor subunits that bind aspartate. This discovery underscores the relevance of aspartate signaling in human lung metastases.
“This correlation emphasizes the clinical significance of our findings,” says Prof. Sarah-Maria Fendt of the VIB-KU Leuven Center for Cancer Biology. “Moreover, existing drugs that target this mechanism could eventually make their way into clinical trials.”
Human samples also demonstrated the broader implications of these findings. The translational program activated by aspartate affects multiple pathways, suggesting that interventions targeting this mechanism might have wide-reaching therapeutic potential.
To confirm their observations, researchers conducted a series of experiments using cultured tumor spheroids—3D clusters of cancer cells—grown in a specialized lung-like medium. This medium mimicked the nutrient composition of lung interstitial fluid and was supplemented with aspartate.
As expected, aspartate supplementation significantly enhanced tumor spheroid growth by increasing eIF5A hypusination and translation-related gene activity.
When researchers silenced the gene responsible for DOHH, aspartate’s effects on cancer growth were blocked. Similarly, silencing eIF5A genes in both human and mouse tumor spheroids halted the growth-promoting effects of aspartate, reinforcing the critical role of this pathway.
This experimental approach highlights the importance of using biologically relevant models to study metastasis. By replicating the lung environment in vitro, researchers were able to uncover mechanisms that might have been overlooked in simpler models.
Aspartate’s dual role as both a biosynthesis metabolite and a signaling molecule in lung metastases opens new avenues for therapeutic intervention. Drugs targeting NMDA receptors or the hypusination pathway could potentially curb the aggressiveness of lung metastases. However, much work remains to translate these findings from the lab to clinical settings.
“These results provide a new perspective on how nutrients influence cancer progression,” explains Doglioni. “By targeting these pathways, we could disrupt the ability of cancer cells to adapt to the lung environment.”
The study also raises questions about whether similar mechanisms exist in metastases to other organs. If so, targeting nutrient-driven signaling pathways could become a broad strategy for combating metastatic disease.
The discovery that aspartate acts as a signaling molecule to boost lung metastases aggressiveness highlights the complexity of cancer biology. While much attention has focused on genetic mutations and immune evasion, this study sheds light on how the biochemical environment of target organs shapes cancer progression.
The findings offer hope for developing targeted therapies that could neutralize the lung’s welcoming environment for cancer cells. By understanding and disrupting these biochemical signals, researchers inch closer to more effective treatments for metastatic cancers.
In the future, integrating these findings into clinical strategies could transform the way metastatic cancer is managed. Aspartate’s role in shaping metastatic niches is a powerful reminder of the intricate interplay between cancer cells and their environment, providing new opportunities to outmaneuver one of medicine’s most formidable foes.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joshua Shavit
Science & Technology Writer | AI and Robotics Reporter
Joshua Shavit is a Los Angeles-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a contributor to The Brighter Side of News, he focuses on positive and transformative advancements in AI, technology, physics, engineering, robotics and space science. Joshua is currently working towards a Bachelor of Science in Business Administration at the University of California, Berkeley. He combines his academic background with a talent for storytelling, making complex scientific discoveries engaging and accessible. His work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.