Scientists discover surprising link between tuberculosis and diabetes
Discover how tuberculosis disrupts liver metabolism, triggers insulin resistance, and heightens diabetes risk, highlighting urgent research priorities.
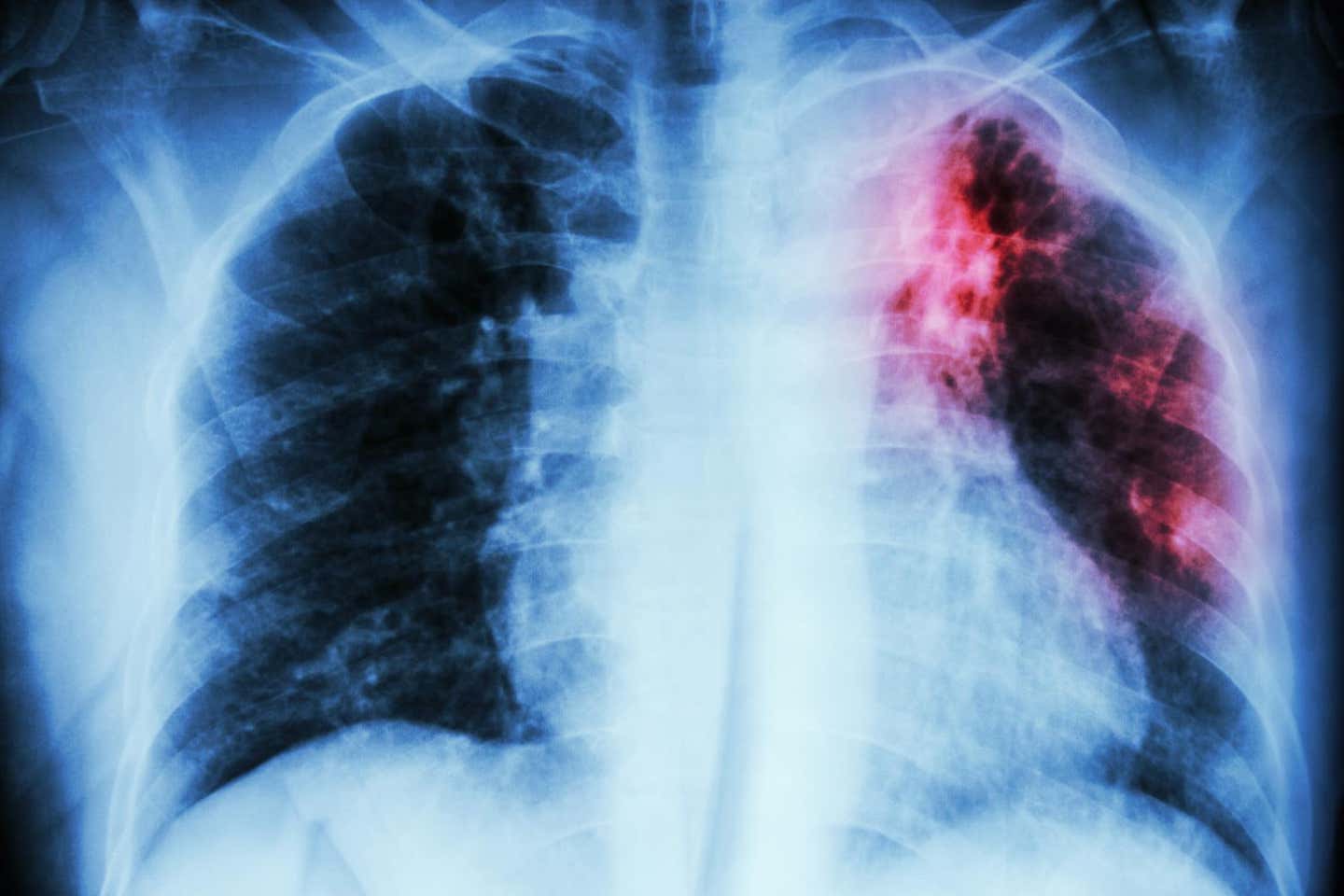
Learn how tuberculosis affects liver function, disrupts glucose metabolism, and links to diabetes, revealing critical insights for future interventions. (CREDIT: PLOS Pathogens)
Tuberculosis (TB) remains a formidable health challenge, claiming over a million lives annually despite advancements in medical care and a deeper understanding of its pathology. Predominantly a pulmonary disease, TB extends its impact far beyond the lungs, manifesting as systemic inflammation and metabolic disturbances that exacerbate patient outcomes.
These complications, ranging from severe fatigue to muscle loss, contribute to treatment failures and increased disability-adjusted life years in regions where TB is endemic. Furthermore, the pervasive burden of undiagnosed and subclinical TB cases underscores the urgency for a comprehensive understanding of the disease’s full systemic effects.
Recent research, published in the journal, PLOS Pathogens, has revealed a complex interplay between TB-induced inflammation and metabolic dysfunctions. While the lung is the primary battleground for Mycobacterium tuberculosis (Mtb), the liver plays a pivotal role as the body’s metabolic regulator.
The liver not only prepares the body to withstand the stress of infection but also supports the metabolically demanding immune response. However, TB disrupts this delicate balance, leading to significant metabolic consequences.
Studies in both animal models and human patients indicate that TB triggers widespread changes in the liver’s metabolic functions. For example, TB patients exhibit reduced levels of cholesterol and albumin, along with elevated hepatic transaminases. Although these changes do not always meet clinical thresholds for liver dysfunction, they highlight TB’s systemic impact.
Researchers have identified a metabolic signature associated with insulin resistance in TB patients, linking the disease to disruptions in glucose metabolism. This finding raises important questions about the long-term health of individuals recovering from TB, as metabolic health is critical for preventing further complications.
The liver’s involvement in systemic metabolism underscores its vulnerability during TB. Chronic inflammation triggered by the disease alters the biochemical pathways that regulate glucose and lipid metabolism. This dysregulation can lead to long-term consequences, including an increased risk of metabolic syndromes such as diabetes and cardiovascular disease. By exploring these connections, scientists hope to uncover therapeutic targets to mitigate these risks and improve patient outcomes.
The interaction between the immune system and metabolic pathways is central to understanding TB’s systemic effects. Type I and type II interferons (IFNs), critical components of the immune response, play contrasting roles. While type II IFN helps control bacterial growth, type I IFN is associated with poorer outcomes.
Related Stories
These immune signals also influence liver function, as seen in studies using TB-infected mice. Researchers observed upregulation of immune-related signaling pathways and downregulation of genes critical for metabolic processes in the liver.
Notably, the liver’s capacity for gluconeogenesis—the production of glucose from non-carbohydrate sources—was significantly impaired. Key enzymes like Pck1 and G6pc were suppressed, and the phosphorylation of CREB, a transcription factor essential for gluconeogenesis, was drastically reduced.
These changes were accompanied by reduced systemic and hepatic insulin sensitivity. Similar patterns were observed in human studies, where metabolic disruptions correlated with TB progression and resolution.
This interplay between immune response and metabolism highlights the complexity of TB’s impact on the body. Immune activation, while essential for controlling infection, places significant metabolic demands on the body. The liver, as the central hub of metabolism, bears the brunt of these demands, leading to systemic metabolic changes that can persist long after the infection is resolved.
The connection between TB and metabolic disorders extends beyond insulin resistance. Chronic inflammation caused by TB can accelerate the development of metabolic diseases like diabetes, which in turn worsens TB outcomes.
This bidirectional relationship has profound implications for public health, particularly in regions with high TB and diabetes prevalence. Late diagnosis of TB may push vulnerable individuals toward metabolic disease, complicating treatment and recovery.
Professor Andrea Cooper from the University of Leicester’s Tuberculosis Research Group highlights this critical link, stating, “Our paper changes the focus from diabetes making TB worse to the possibility that late diagnosis of TB can contribute to disruption of glucose metabolism, insulin resistance, and therefore can promote progress towards diabetes in those that are susceptible.”
These findings underscore the need for metabolic screening in TB drug and vaccine trials. Such measures could identify at-risk individuals early and mitigate the cascading effects of TB-induced metabolic dysfunction. Additionally, these insights open up possibilities for integrating metabolic health assessments into TB care protocols, ensuring that patients receive comprehensive treatment addressing both infectious and metabolic concerns.
Beyond diabetes, TB’s impact on metabolic health has broader implications. The disease’s systemic nature suggests that other organs, including the heart and kidneys, may also experience stress during infection. Understanding these interconnected effects is vital for developing holistic treatment approaches that address the full spectrum of TB’s impact on the body.
The discovery that TB alters liver metabolism opens new avenues for therapeutic interventions. Researchers aim to define the molecular pathways through which the immune response modifies liver function. By understanding these mechanisms, scientists hope to develop targeted strategies to address the metabolic consequences of TB. Such interventions could improve patient outcomes and reduce the risk of developing secondary metabolic diseases.
Dr. Mrinal Das, a leading researcher in the study, reanalyzed human metabolic data to confirm that liver glucose metabolism is disrupted during TB progression.
This work complements findings from laboratory models, which showed that TB triggers immune responses within liver cells and alters glucose metabolism. The implications of these discoveries extend to latent TB, where subclinical infections may silently impact metabolic health.
Prevention through improved vaccines remains a priority for the World Health Organization. Currently, only one vaccine is available, primarily for infants and young children. Advances in understanding TB’s systemic effects could guide the development of more effective vaccines and treatments.
By addressing both the infectious and metabolic dimensions of TB, researchers aim to reduce its global burden and improve the quality of life for millions affected by the disease.
Professor Cooper emphasizes the importance of this integrated approach: “Our future aim is to define the molecular pathways by which the immune response is changing liver metabolism, allowing us to potentially create targeted interventions.” Such efforts could pave the way for a new era in TB management, where metabolic health becomes a cornerstone of comprehensive care.
The need for interdisciplinary research has never been more pressing. The integration of immunology, metabolism, and clinical care holds promise for transforming TB treatment. By focusing on the broader systemic effects of the disease, scientists and clinicians can work together to develop innovative solutions that go beyond traditional approaches.
From vaccine development to therapeutic interventions, the insights gained from this research could reshape the global fight against TB and its associated health challenges.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joshua Shavit
Science & Technology Writer | AI and Robotics Reporter
Joshua Shavit is a Los Angeles-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a contributor to The Brighter Side of News, he focuses on positive and transformative advancements in AI, technology, physics, engineering, robotics and space science. Joshua is currently working towards a Bachelor of Science in Business Administration at the University of California, Berkeley. He combines his academic background with a talent for storytelling, making complex scientific discoveries engaging and accessible. His work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.