Researchers cook up a new way to remove microplastics from water
Researchers at Princeton Engineering have found a way to turn your breakfast food into a new material that can cheaply remove microplastics.
[Nov 22, 2022: Steven Schultz, Princeton University, Engineering School]
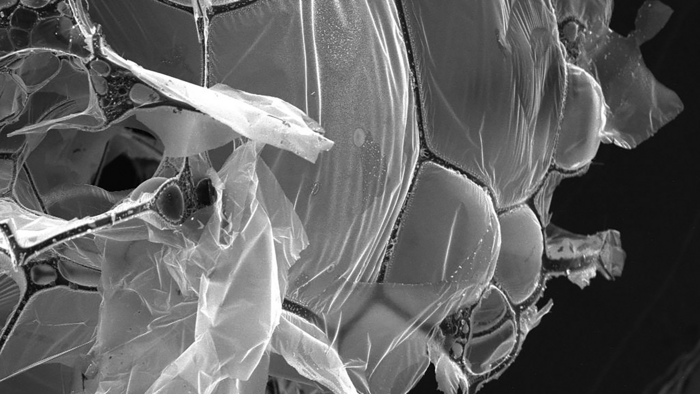
The structure of the aerogel is formed by graphene sheets stretched across carbon fiber networks. (Credit: Shaharyar Wani)
Researchers at Princeton Engineering have found a way to turn your breakfast food into a new material that can cheaply remove salt and microplastics from seawater.
The researchers used egg whites to create an aerogel, a lightweight and porous material that can be used in many types of applications, including water filtration, energy storage, and sound and thermal insulation. Craig Arnold, the Susan Dod Brown Professor of Mechanical and Aerospace Engineering and vice dean of innovation at Princeton, works with his lab to create new materials, including aerogels, for engineering applications.
One day, sitting in a faculty meeting, he had an idea.
“I was sitting there, staring at the bread in my sandwich,” said Arnold. “And I thought to myself, this is exactly the kind of structure that we need.” So he asked his lab group to make different bread recipes mixed with carbon to see if they could recreate the aerogel structure he was looking for. None of them worked quite right initially, so the team kept eliminating ingredients as they tested, until eventually only egg whites remained.
Egg whites are a complex system of almost pure protein that — when freeze-dried and heated to 900 degrees Celsius in an environment without oxygen — create a structure of interconnected strands of carbon fibers and sheets of graphene.
Related Stories
In a paper published in Materials Today, Arnold and his coauthors showed that the resulting material can remove salt and microplastics from seawater with 98% and 99% efficiency, respectively.
“The egg whites even worked if they were fried on the stove first, or whipped,” said Sehmus Ozden, first author on the paper. Ozden is a former postdoctoral research associate at the Princeton Center for Complex Materials and now a scientist at Aramco Research Center. While regular store-bought egg whites were used in initial tests, Ozden said, other similar commercially available proteins produced the same results.
“Eggs are cool because we can all connect to them and they are easy to get, but you want to be careful about competing against the food cycle,” said Arnold.
Figure 1. (a) Schematic synthesis illustration of the hierarchical monolith of graphitic carbon and carbon fiber (G-CF) aerogel network, (b) picture of ultralightweight multifunctional G-CF aerogel, (c, d) SEM images of the G-CF aerogel surface and (e) inner picture of ultralightweight G-CF aerogel (f, g). SEM images of the porous inner structure of the G-CF aerogel consist of buckyball-like cages. (CREDIT: Materials Today)
Because other proteins also worked, the material can potentially be produced in large quantities relatively cheaply and without impacting the food supply. One next step for the researchers, Ozden noted, is refining the fabrication process so it can be used in water purification on a larger scale.
If this challenge can be solved, the material has significant benefits because it is inexpensive to produce, energy-efficient to use and highly effective. “Activated carbon is one of the cheapest materials used for water purification. We compared our results with activated carbon, and it’s much better,” said Ozden. Compared with reverse osmosis, which requires significant energy input and excess water for operation, this filtration process requires only gravity to operate and wastes no water.
Figure 3. Evolution of the hierarchical G-CF structure during the process. (a) Microscopic images of the EW proteins transform at different temperatures, (b) simulation of heating and pyrolysis of the β-sheet model, (c) the Raman spectra of EW in the D and G band region at different temperatures, (d) the XRD spectra of EW protein transformation at different temperatures and (e) simulation of pyrolysis of the stacked sheet model (ii) for the formation of G and CF. (CREDIT: Materials Today)
While Arnold sees water purity as a “major grand challenge,” that is not the only potential application for this material. He is also exploring other uses related to energy storage and insulation.
The research included contributions from the departments of chemical and biological engineering and geosciences at Princeton and elsewhere. “It’s one thing to make something in the lab,” said Arnold, “and it’s another thing to understand why and how.” Collaborators who helped answer the why and how questions included professors Rodney Priestley and A. James Link from chemical and biological engineering, who helped identify the transformation mechanism of the egg white proteins at the molecular level. Princeton colleagues in geosciences assisted with measurements of water filtration.
The integration of 2D-graphitic carbon (G) with 1D-carbon nanofiber (CF) allows for the unique properties of 2D graphitic carbon to be combined. (CREDIT: Materials Today)
Susanna Monti of the Institute for Chemistry of Organometallic Compounds and Valentina Tozzi from Instituto Nanoscienze and NEST-Scuola Normale Superiore created the theoretical simulations that revealed the transformation of egg white proteins into the aerogel.
The article, “Egg protein derived ultralightweight hybrid monolithic aerogel for water purification,” was published in the journal Materials Today. Besides Arnold, Monti, Ozden, Priestley, Link and Tozzi, authors include Nikita Dutta, a former graduate student in mechanical and aerospace engineering who is now at the National Renewable Energy Laboratory; Stefania Gill, John Higgins and Nick Caggiano of Princeton University; and Nicola Pugno of the University of Trento and Queen Mary University of London. Support was provided in part by the Princeton Center for Complex Materials and the U.S. National Science Foundation.
For more environmental news stories check out our Green Impact section at The Brighter Side of News.
Note: Materials provided above by Princeton University, Engineering School. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.
Joshua Shavit
Science & Technology Writer | AI and Robotics Reporter
Joshua Shavit is a Los Angeles-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a contributor to The Brighter Side of News, he focuses on positive and transformative advancements in AI, technology, physics, engineering, robotics and space science. Joshua is currently working towards a Bachelor of Science in Business Administration at the University of California, Berkeley. He combines his academic background with a talent for storytelling, making complex scientific discoveries engaging and accessible. His work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.