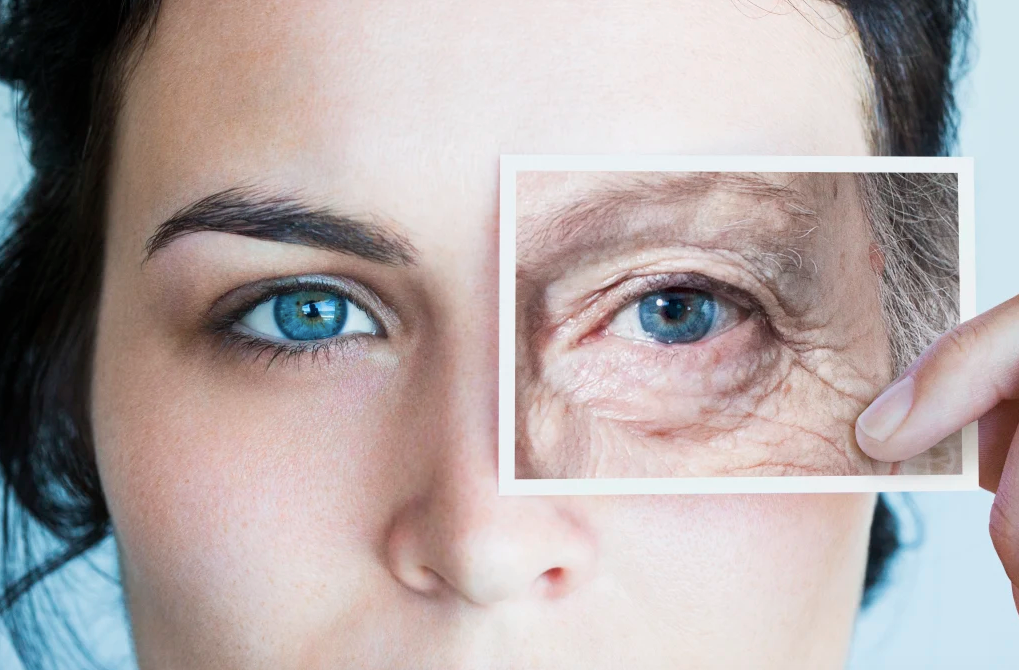
Protein discovery unlocks the secret to reversing muscle aging
Researchers at the University at Buffalo have unveiled a groundbreaking discovery that may hold the key to reversing cellular aging.

The study’s findings offer a promising avenue for combating the age-related deterioration of skeletal muscle cells. (CREDIT: Creative Commons)
In the relentless pursuit to slow or even reverse the aging process, scientists at the University of Buffalo have made a groundbreaking discovery. This new study, published in the renowned journal Science Advances, centers around a protein named NANOG, drawing its name from the mythical Irish land of Tír na nÓg, a place steeped in legends of eternal youth and vitality.
Their research offers a promising path to combat the age-related decline of skeletal muscle cells. By overexpressing NANOG in senescent myoblasts—the embryonic precursors to muscle tissue—the research team achieved a remarkable reversal of key cellular aging markers. These improvements were observed in autophagy, energy homeostasis, genomic stability, nuclear integrity, and mitochondrial function.
One of the most striking results was the increase in muscle stem cells in prematurely aging mice, showcasing the potential of rejuvenating aging cells within the body. Importantly, this approach avoids the need to reprogram cells to an embryonic pluripotent state, a process often associated with tumor risks in stem cell therapy.
Dr. Stelios T. Andreadis, the study's corresponding author and a SUNY Distinguished Professor in the Department of Chemical and Biological Engineering at the UB School of Engineering and Applied Sciences, explained the broader goals of their research: "Our work focuses on understanding the mechanisms of NANOG’s actions in hopes of discovering druggable targets in signaling or metabolic networks that mimic the anti-aging effects of NANOG. Ultimately, the work could help lead to new treatments or therapies that help reverse cellular senescence, and aid the many people suffering from age-related disorders."
Understanding Cellular Senescence and Its Impact
Cellular senescence is a critical factor in aging and age-related diseases. Senescent cells are unique in that they stop dividing but don’t die when they should. Instead, they linger and secrete chemicals that can cause inflammation. Similar to a single moldy fruit spoiling the entire batch, a few senescent cells can spread inflammation and damage to surrounding healthy cells.
Interestingly, not all senescent cells are harmful. The compounds they release, known as the senescent secretome, play important roles throughout life, such as during embryonic development, childbirth, and wound healing.
However, as we age, the number of senescent cells increases. The immune system's efficiency declines over time, allowing these cells to accumulate and affect nearby healthy cells. This accumulation impacts the body's ability to handle stress, recover from injuries, and maintain cognitive functions, as senescent cells in the brain can impair learning and memory.
Senescence has been linked to numerous age-related conditions, including cancer, diabetes, osteoporosis, cardiovascular disease, stroke, Alzheimer's disease, and related dementias, as well as osteoarthritis. It also contributes to the decline in vision, mobility, and cognitive abilities.
Moreover, researchers suggest that senescent skin cells may be behind the sagging and wrinkling of aging skin. They also play a role in the severe inflammation seen in older adults with COVID-19, known as the cytokine storm.
The Role of NANOG in Combating Aging
The discovery of NANOG’s powerful anti-aging properties is a significant breakthrough in the study of cellular senescence. By overexpressing this protein in senescent myoblasts, the researchers were able to reverse many aging hallmarks, offering hope for combating age-related disorders without the risks associated with reprogramming cells to an embryonic state.
One key improvement was in autophagy, the cell’s internal cleaning system, which ensures that damaged cellular components are efficiently removed. Energy homeostasis, essential for maintaining cellular functions, was also restored to a youthful state. Additionally, genomic stability, nuclear integrity, and mitochondrial function—all critical for cell health—showed marked improvements.
A particularly promising result was the increase in muscle stem cells in prematurely aging mice. This finding suggests potential future therapies not only for muscle cells but possibly for other tissues and organs. Developing targeted drugs that mimic NANOG’s effects opens exciting possibilities for treating a wide range of age-related disorders.
The Future of Anti-Aging Research
As the scientific community delves deeper into the mechanisms of cellular senescence and NANOG’s rejuvenating effects, the potential for new anti-aging treatments becomes more tangible. While we are still unraveling the complexities of aging at the cellular level, this discovery is a significant step toward understanding longevity and vitality.
The potential for harnessing NANOG and similar proteins as therapies for age-related diseases underscores human ingenuity and our relentless pursuit of healthier, longer lives. In the near future, we might see revolutionary treatments that can turn back the clock on cellular aging, offering hope to countless individuals burdened by age-related conditions.
The mythical land of Tír na nÓg, symbolizing eternal youth, may soon move from legend to reality, thanks to scientific progress. This journey toward harnessing NANOG and similar proteins is a testament to the remarkable advances in our understanding of aging and the potential for groundbreaking treatments.
As research progresses, the dream of finding effective anti-aging treatments that can improve quality of life for many is becoming more attainable. This study represents a significant milestone in the quest for longevity and health, bringing us closer to a future where age-related decline can be effectively managed and perhaps even reversed.
Note: Materials provided above by the The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.
Joseph Shavit
Head Science News Writer | Communicating Innovation & Discovery
Based in Los Angeles, Joseph Shavit is an accomplished science journalist, head science news writer and co-founder at The Brighter Side of News, where he translates cutting-edge discoveries into compelling stories for a broad audience. With a strong background spanning science, business, product management, media leadership, and entrepreneurship, Joseph brings a unique perspective to science communication. His expertise allows him to uncover the intersection of technological advancements and market potential, shedding light on how groundbreaking research evolves into transformative products and industries.