New ‘smart’ immune cells continuously hunt and kill cancer cells
EchoBack CAR T-cells, guided by ultrasound, offer a longer-lasting and safer cancer treatment by avoiding damage to healthy tissue.
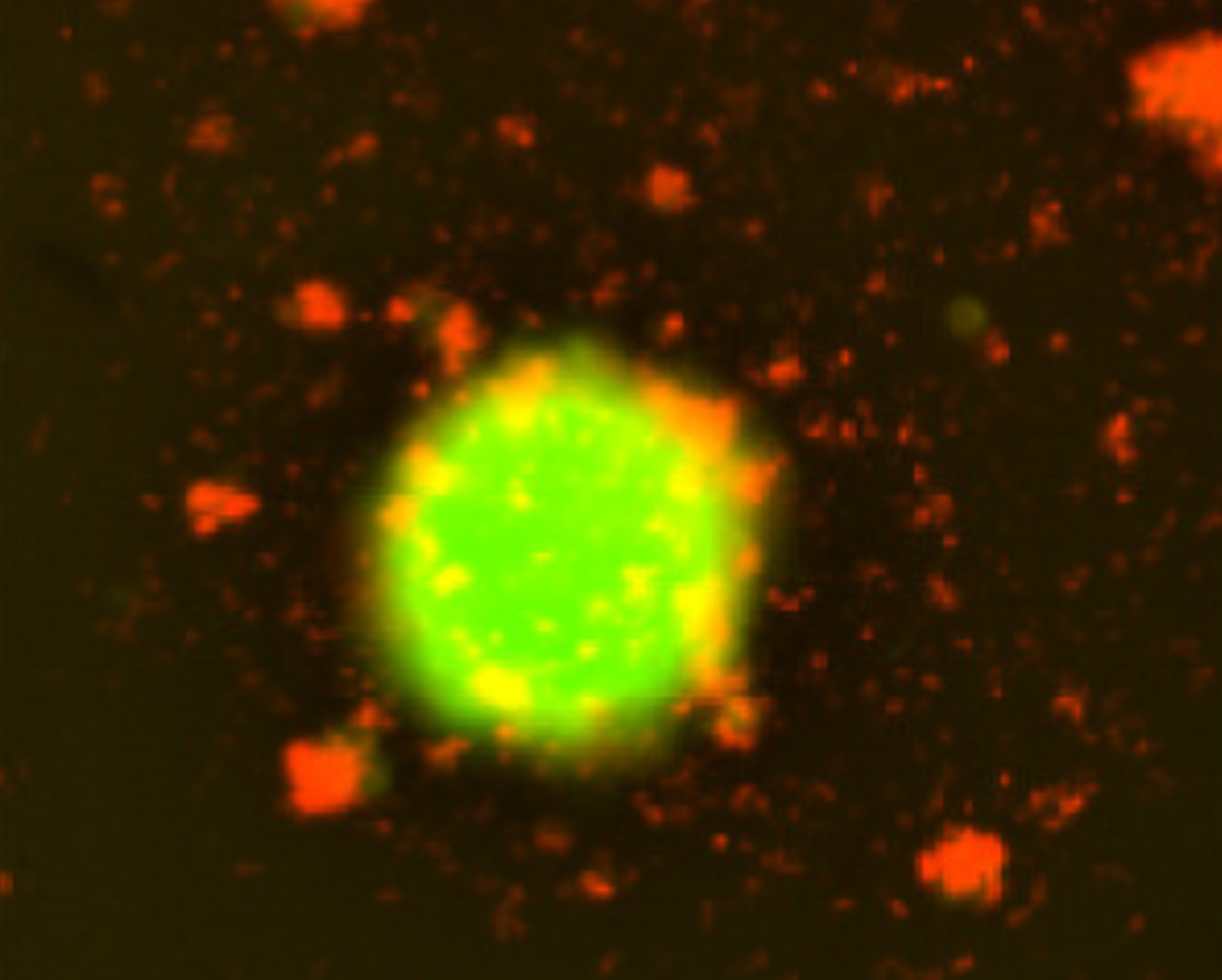
Representative fluorescent images showing tumor spheroids (green, EGFP+) and EchoBack-CAR T cells (red, mCherry+). (CREDIT: Cell)
Researchers have unlocked a powerful new way to fight solid tumors using smart immune cells that stay active longer and hit harder. Called EchoBack CAR T-cells, these new cancer-fighting tools work with a quick burst of focused ultrasound to destroy tumors while avoiding damage to healthy tissues.
This breakthrough is the result of work by biomedical engineers at the University of Southern California. Their new design improves chimeric antigen receptor (CAR) T-cell therapy — a promising but limited treatment method that has mostly worked for blood cancers like leukemia. With this new version, the team has found a way to bring those same benefits to harder-to-treat solid tumors.
A Smarter Immune Weapon
CAR T-cell therapy reprograms your immune cells to recognize and attack cancer. It begins by collecting your T-cells, altering their DNA in a lab, and returning them to your body ready to find and kill cancer cells.
This strategy has cured some forms of leukemia and lymphoma. But with solid tumors like brain, prostate, or breast cancer, the treatment faces serious problems. T-cells can get too tired, known as exhaustion, or they may start attacking healthy tissues, a danger called on-target off-tumor toxicity. They also often vanish from the body too quickly to finish the job.
Scientists have been working to fix those flaws. Dr. Peter Yingxiao Wang, who leads the USC Alfred E. Mann Department of Biomedical Engineering, and his team found a way to take things a step further. Their lab built CAR T-cells that don't just attack cancer — they do it smarter, safer, and for longer.
“We call them EchoBack CAR T-cells because they echo the ultrasound stimulation that turns them on,” said Dr. Wang.
Controlled by Sound, Powered by Feedback
The team’s approach uses focused ultrasound, a harmless and noninvasive sound wave, as a trigger. A short 10-minute pulse acts as an “on switch” for the immune cells. Once activated, the cells stay aggressive against tumors for up to five days — far longer than older versions that lasted just 24 hours.
“Patients using earlier versions may need daily treatment,” said Longwei Liu, lead author and assistant professor at USC’s Viterbi School of Engineering. “With EchoBack, treatments could happen once every two weeks or even less often.”
Related Stories
The secret lies in the combination of ultrasound control and a powerful positive feedback loop. Once activated, the cells recognize nearby cancer cells and ramp up their own tumor-killing ability — without constant external control. This makes them both persistent and precise. And when the tumor is gone, they naturally wind down, reducing the risk of harm to healthy tissue.
“Whenever there is a tumor cell nearby, our CAR T-cell senses it and produces more killing molecules,” Liu explained. “If the cell leaves the tumor area, the killing function fades. That’s why it’s safe — the CAR T-cells know where to work and when to stop.”
Built from the Ground Up
Creating EchoBack cells took more than just smart ideas. It required deep knowledge of how genes turn on and off. To control the cells with sound, the team engineered them with a highly sensitive heat-shock promoter. This is a segment of DNA that reacts to tiny changes in temperature caused by the ultrasound.
They didn’t just guess at the design. Instead, they screened hundreds of potential promoters using a special library of gene sequences, searching for the one that responded best to sound. Once they found the winner, they added a self-boosting loop tied to the CAR T-cell’s own internal signaling systems — which include calcium, ZAP70 kinase, MAPK/ERK, NFAT, and NF-κB pathways.
This combination allows a brief ultrasound pulse to launch a strong and long-lasting immune attack that continues even after the stimulation ends.
In earlier models, CAR expression dropped off quickly, making the therapy less effective. But this design keeps CAR molecules active longer, without constant outside input. And it gives researchers a clear path to tuning how much or how little immune activity is triggered.
Safe and Strong Against Solid Tumors
To test their invention, the research team targeted a brain cancer called glioblastoma using EchoBack cells tuned to attack GD2, a protein found on those tumors. They also tested a version for prostate cancer that focused on PSMA.
In 3D tumor models and in live mice, the EchoBack CAR T-cells showed impressive results. They wiped out tumors better than standard CAR T-cells and avoided damage to healthy tissue. No off-tumor toxicity was seen in the brain, heart, or lungs — organs that have caused problems in past trials.
The team ran single-cell RNA sequencing to study how individual T-cells behaved. Results showed EchoBack cells stayed stronger, killed better, and didn’t tire as quickly.
“We challenged both types of CAR T-cells with tumor cells,” Liu said. “Standard CAR cells were exhausted and dysfunctional. Our ultrasound-controlled ones stayed active and killed more cancer.”
From Lab to Clinic
USC PhD students Peixiang He and Yuxuan Wang played key roles in the project, along with help from researchers at Yale and the University of North Carolina at Chapel Hill. Qifa Zhou, USC’s Zohrab A. Kaprielian Fellow in Engineering, also helped develop the ultrasound system.
Together, they’ve created a real, working system that could soon enter clinical trials.
“This isn’t just a concept,” Liu said. “These smart CAR T-cells actually listen to the ultrasound and sense the tumor cells. They’ve never been developed before, and we believe they can bring hope to patients with tumors that don’t respond to other treatments.”
Because the system is modular, it can be adapted to treat other cancers. Breast cancer, retinoblastoma, and even more types of hard-to-reach solid tumors may one day be targeted by EchoBack cells.
“It’s definitely a breakthrough,” said Dr. Wang. “This makes ultrasound-controllable CAR T-cell therapy practical for real medical use.”
Looking Forward
EchoBack CAR T-cells blend synthetic biology, genetics, and sound-based control in a way that could change how cancer is treated. The work builds on earlier ideas like sonogenetics — where light or sound can control cells — but overcomes a key hurdle: short-term activation.
By finding a promoter that stays active longer, and combining it with feedback from the CAR system itself, the researchers have created a tool that works with a brief signal but keeps going on its own. That could mean safer, more effective, and less frequent treatments for people with some of the toughest cancers.
And because the system naturally stops when the cancer cells are gone, the chance of attacking healthy cells is reduced. This built-in safety could help bring CAR T-cell therapy to many more patients.
“These types of CAR T-cells have never been developed previously,” Liu said. “We’re looking forward to what they can do for patients in the future.”
Research findings are available online in the journal Cell.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joshua Shavit
Science & Technology Writer | AI and Robotics Reporter
Joshua Shavit is a Los Angeles-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a contributor to The Brighter Side of News, he focuses on positive and transformative advancements in AI, technology, physics, engineering, robotics and space science. Joshua is currently working towards a Bachelor of Science in Business Administration at the University of California, Berkeley. He combines his academic background with a talent for storytelling, making complex scientific discoveries engaging and accessible. His work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.



