New shingles vaccine significantly reduces dementia risk
New research finds shingles vaccine significantly cuts dementia risk by 20%, providing fresh hope for dementia prevention.

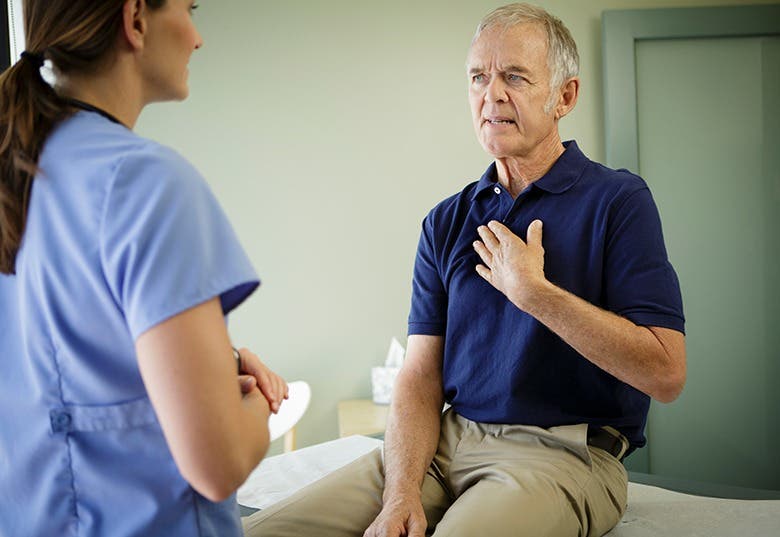
A groundbreaking study from Wales reveals the shingles vaccine may dramatically reduce dementia risk. (CREDIT: CC BY-SA 4.0)
For decades, researchers have searched for a way to slow or prevent dementia. Despite countless studies, solutions remain scarce. But now, a common shingles vaccine might provide an unexpected answer.
In a groundbreaking study involving health records from Wales, researchers discovered that adults who received the herpes zoster (shingles) vaccine were 20% less likely to develop dementia. The findings, published in Nature, are among the strongest evidence yet linking vaccination with dementia prevention.
Understanding Shingles and Dementia
Shingles and dementia may seem unrelated, but a hidden connection exists. Shingles occurs when the virus responsible for chickenpox, varicella-zoster, reactivates later in life. This virus can lie dormant for decades within nerve cells. As the immune system weakens with age, the virus awakens, causing a painful rash and potential nerve damage.
Dementia, on the other hand, is a degenerative brain condition affecting memory, thinking, and behavior. Worldwide, over 55 million people suffer from dementia, with nearly 10 million new cases annually. Alzheimer’s, the most common form, has long been linked to brain plaques and tangles. However, recent studies suggest viruses, like herpesviruses, might also contribute to dementia.
Previous research connected herpesvirus infections to dementia risk, raising the question of whether controlling these viruses might delay or even prevent dementia onset.
A Unique Public Health Experiment
Researchers had struggled to establish a clear link between vaccinations and dementia because vaccinated individuals often differ significantly from unvaccinated ones. People who seek vaccinations usually have healthier lifestyles, which could independently reduce dementia risk.
Related Stories
“All these associational studies suffer from the basic problem that people who get vaccinated have different health behaviors than those who don’t,” explained senior researcher Pascal Geldsetzer, MD, PhD, and assistant professor of medicine at Stanford Medicine. Such differences make it difficult to confirm vaccines' true impact.
However, an unusual vaccination policy in Wales provided an ideal testing ground. Starting in 2013, the Welsh government introduced the shingles vaccine for adults based on a strict birthdate rule. People turning 80 just before September 2, 2013, were permanently ineligible. Those turning 80 right after that date could receive the vaccine for at least one year. This small age difference created two comparable groups—essentially identical, except for vaccination eligibility.
“We know that if you take a thousand people born in one week and a thousand people born a week later, there shouldn’t be anything different about them on average,” Geldsetzer said. “They are similar to each other apart from this tiny difference in age.”
Using electronic health records from over 280,000 people aged between 71 and 88, researchers compared dementia rates over seven years. They specifically examined people who were within one week of the cutoff date, ensuring the groups differed only by vaccine eligibility.
Clear Evidence Emerges
Initially, the difference in vaccination rates was dramatic. Among those born just a week after the cutoff, 47.2% received the shingles vaccine, compared to almost none born one week before. Over seven years, these vaccination rates translated into significant health outcomes.
As expected, shingles infections were substantially lower in vaccinated adults—about 37% fewer cases. Remarkably, dementia diagnoses also dropped by 3.5 percentage points, a relative reduction of approximately 20%.
“It was a really striking finding,” Geldsetzer noted. “This huge protective signal was there, any which way you looked at the data.”
The researchers carefully analyzed other health factors that might have influenced these results. Education levels, previous health conditions, and participation in other preventive health programs were identical in both groups. This indicated that vaccination itself—rather than hidden factors—reduced dementia risk.
Additionally, they confirmed these results using alternative measures. They analyzed death certificates for dementia-related deaths, providing another layer of validation. The reduction remained consistent.
Stronger Protection for Women
Interestingly, the vaccine offered even greater protection for women. Women generally respond more robustly to vaccines, producing stronger immune responses. They are also more prone to shingles infections. This suggests the vaccine might provide stronger benefits for women, though further research is necessary to understand why.
Scientists still aren't sure exactly how the shingles vaccine protects against dementia. One theory is that by preventing shingles outbreaks, the vaccine reduces chronic inflammation and nerve damage, which could accelerate dementia. Another possibility is the vaccine broadly boosts immune health, helping the body fight other dementia-causing pathogens.
Researchers also considered whether healthcare patterns changed after shingles infections. They found no evidence that dementia diagnoses resulted merely from increased medical attention after shingles. The vaccination itself seemed key.
Confirming Results Around the World
The team has replicated these encouraging findings using health records from other countries, including England, Australia, Canada, and New Zealand. In each case, similar vaccination policies led to comparable dementia reductions.
“We just keep seeing this strong protective signal for dementia in dataset after dataset,” Geldsetzer said.
Despite compelling evidence, questions remain. The vaccine used in this study contained a live-attenuated (weakened) virus, no longer widely available since newer vaccines replaced it. Researchers want to know if newer vaccines, like Shingrix, which contain only viral proteins and are more effective against shingles, also protect against dementia.
Geldsetzer hopes to secure funding for a large-scale clinical trial to confirm these results conclusively. A randomized controlled trial—assigning people to receive the vaccine or a placebo—would provide definitive proof.
“It would be a very simple, pragmatic trial because we have a one-off intervention that we know is safe,” he emphasized. Results from such a trial could become apparent within just a few years, accelerating dementia prevention strategies.
Promising Implications for Public Health
If confirmed, these findings could reshape how doctors approach dementia prevention. Vaccinations might become routine not just for their intended purpose but also for their unexpected benefits. This could significantly reduce dementia rates globally, especially considering dementia currently lacks effective preventive measures.
“At least investing a subset of our resources into investigating these pathways could lead to breakthroughs in terms of treatment and prevention,” Geldsetzer concluded.
The shingles vaccine, initially aimed at preventing a painful rash, may now offer hope against one of the world’s most feared diseases.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Rebecca Shavit
Science & Technology Journalist | Innovation Storyteller
Based in Los Angeles, Rebecca Shavit is a dedicated science and technology journalist who writes for The Brighter Side of News, an online publication committed to highlighting positive and transformative stories from around the world. With a passion for uncovering groundbreaking discoveries and innovations, she brings to light the scientific advancements shaping a better future. Her reporting spans a wide range of topics, from cutting-edge medical breakthroughs and artificial intelligence to green technology and space exploration. With a keen ability to translate complex concepts into engaging and accessible stories, she makes science and innovation relatable to a broad audience.