New injectable therapy can reverse paralysis and repair spinal cord injuries
Researchers have developed a new injectable therapy that harnesses “dancing molecules” to reverse paralysis and repair spinal cord tissue
[May. 3, 2023: RS Shavit, The Brighter Side of News]
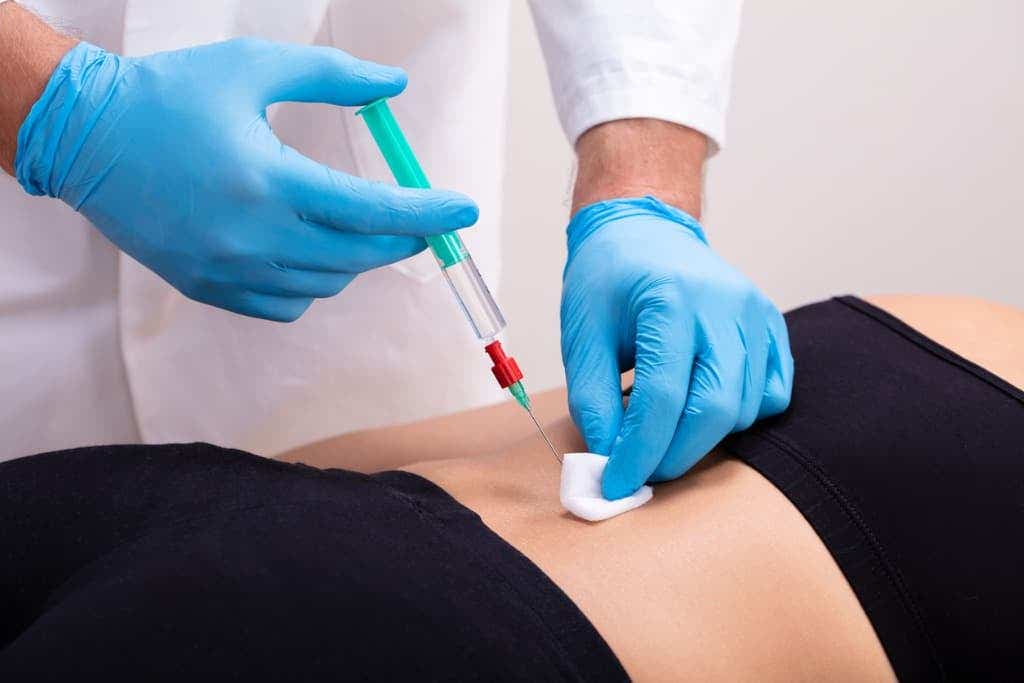
Researchers have developed a new injectable therapy that harnesses “dancing molecules” to reverse paralysis and repair tissue after severe spinal cord injuries. (CREDIT: Creative Commons)
Northwestern University researchers have developed a new injectable therapy that harnesses "dancing molecules" to reverse paralysis and repair tissue after severe spinal cord injuries.
The breakthrough therapy aims to prevent individuals from becoming paralyzed after major trauma or disease. Currently, there are no therapeutics that trigger spinal cord regeneration.
The research is published in the journal Science.
The research team administered a single injection to the tissues surrounding the spinal cords of paralyzed mice. Just four weeks later, the animals regained the ability to walk. By sending bioactive signals to trigger cells to repair and regenerate, the breakthrough therapy dramatically improved severely injured spinal cords in five key ways.
The therapy biodegrades into nutrients for the cells within 12 weeks and then disappears completely from the body without noticeable side effects.
Related Stories
The therapy is the first study in which researchers controlled the collective motion of molecules through changes in chemical structure to increase a therapeutic's efficacy. "Our research aims to find a therapy that can prevent individuals from becoming paralyzed after major trauma or disease," said Northwestern's Samuel I. Stupp, who led the study.
According to the National Spinal Cord Injury Statistical Center, nearly 300,000 people are currently living with a spinal cord injury in the United States. Life for these patients can be extraordinarily difficult. Less than 3% of people with complete injury ever recover basic physical functions. Life expectancy for people with spinal cord injuries is significantly lower than people without spinal cord injuries and has not improved since the 1980s.
The secret behind Stupp's new breakthrough therapeutic is tuning the motion of molecules so that they can find and properly engage constantly moving cellular receptors. Injected as a liquid, the therapy immediately gels into a complex network of nanofibers that mimic the extracellular matrix of the spinal cord. By matching the matrix's structure, mimicking the motion of biological molecules, and incorporating signals for receptors, the synthetic materials can communicate with cells.
This GIF shows a side-by-side comparison of an untreated mouse next to a mouse treated with Northwestern's injectable therapeutic. (CREDIT: Northwestern University)
Once connected to the receptors, the moving molecules trigger two cascading signals, both of which are critical to spinal cord repair. One signal prompts the long tails of neurons in the spinal cord, called axons, to regenerate. Severing or damaging axons can result in the loss of feeling in the body or even paralysis.
The second signal helps neurons survive after injury because it causes other cell types to proliferate, promoting the regrowth of lost blood vessels that feed neurons and critical cells for tissue repair. The therapy also induces myelin to rebuild around axons and reduces glial scarring, which acts as a physical barrier that prevents the spinal cord from healing.
A new injectable therapy forms nanofibers with two different bioactive signals (green and orange) that communicate with cells to initiate repair of the injured spinal cord. (CREDIT: Mark Seniw)
Stupp is Board of Trustees Professor of Materials Science and Engineering, Chemistry, Medicine and Biomedical Engineering at Northwestern, where he is founding director of the Simpson Querrey Institute for BioNanotechnology (SQI) and its affiliated research center, the Center for Regenerative Nanomedicine. He has appointments in the McCormick School of Engineering, Weinberg College of Arts and Sciences, and Feinberg School of Medicine.
Stupp and his team found that fine-tuning the molecules' motion within the nanofiber network to make them more agile resulted in greater therapeutic efficacy in paralyzed mice. They also confirmed that formulations of their therapy with enhanced molecular motion performed better during in vitro tests with human cells, indicating increased bioactivity and cellular signaling.
"Given that cells themselves and their receptors are in constant motion, you can imagine that molecules moving more rapidly would encounter these receptors more often," Stupp said. "If the molecules are sluggish and not as 'social,' they may never come into contact with the cells."
The therapy biodegrades into nutrients for the cells within 12 weeks and then disappears completely from the body without noticeable side effects. Researchers tested this therapy on mice and observed that it regenerated the severed extensions of neurons, called axons, in the mice's spinal cords. This was a major breakthrough in the field of regenerative medicine, as previously there were no effective treatments for repairing nerve damage in the spinal cord.
Depicted here is a longitudinal spinal cord section treated with the most bioactive therapeutic scaffold, captured 12 weeks after injury. Blood vessels (red) regenerated within the lesion. Laminin is stained in green and cells are stained in blue. (CREDIT: Northwestern University)
Excited by these promising results, the researchers began working on developing a version of the therapy for human use. They conducted extensive safety testing and clinical trials, which confirmed that the therapy was safe and effective in humans as well.
As word spread about this groundbreaking new therapy, patients with spinal cord injuries began lining up to receive the treatment. The first human trials were a resounding success, with many patients experiencing significant improvements in their mobility and sensory function.
Over the next few years, the therapy became widely available, and it soon became the standard of care for patients with spinal cord injuries. As a result, many people who were previously paralyzed or severely disabled were able to regain their independence and lead more fulfilling lives.
By mutating the peptide sequence of the amphiphilic monomers in nonbioactive domains, researchers intensified the motions of molecules within scaffold fibrils. (CREDIT: Science)
The success of this therapy also inspired further research into other applications of regenerative medicine, and in time, researchers were able to develop similar treatments for a range of other conditions, including heart disease, liver failure, and even certain types of cancer.
Looking back, it's clear that the discovery of this therapy was a major turning point in the history of medicine, and it opened up new avenues for treating previously untreatable conditions. And while there is still much work to be done, the future looks bright for regenerative medicine and the millions of people it has the potential to help.
Other Northwestern study authors include Evangelos Kiskinis, assistant professor of neurology and neuroscience in Feinberg; research technician Feng Chen; postdoctoral researchers Ivan Sasselli, Alberto Ortega and Zois Syrgiannis; and graduate students Alexandra Kolberg-Edelbrock, Ruomeng Qiu and Stacey Chin. Peter Mirau of the Air Force Research Laboratories and Steven Weigand of Argonne National Laboratory also are co-authors.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.
Joseph Shavit
Head Science News Writer | Communicating Innovation & Discovery
Based in Los Angeles, Joseph Shavit is an accomplished science journalist, head science news writer and co-founder at The Brighter Side of News, where he translates cutting-edge discoveries into compelling stories for a broad audience. With a strong background spanning science, business, product management, media leadership, and entrepreneurship, Joseph brings a unique perspective to science communication. His expertise allows him to uncover the intersection of technological advancements and market potential, shedding light on how groundbreaking research evolves into transformative products and industries.



