New connections between obesity and heart failure revealed
New study has revealed the impact of obesity on muscle structure in patients having a form of heart failure called heart failure with a preserved ejection fraction
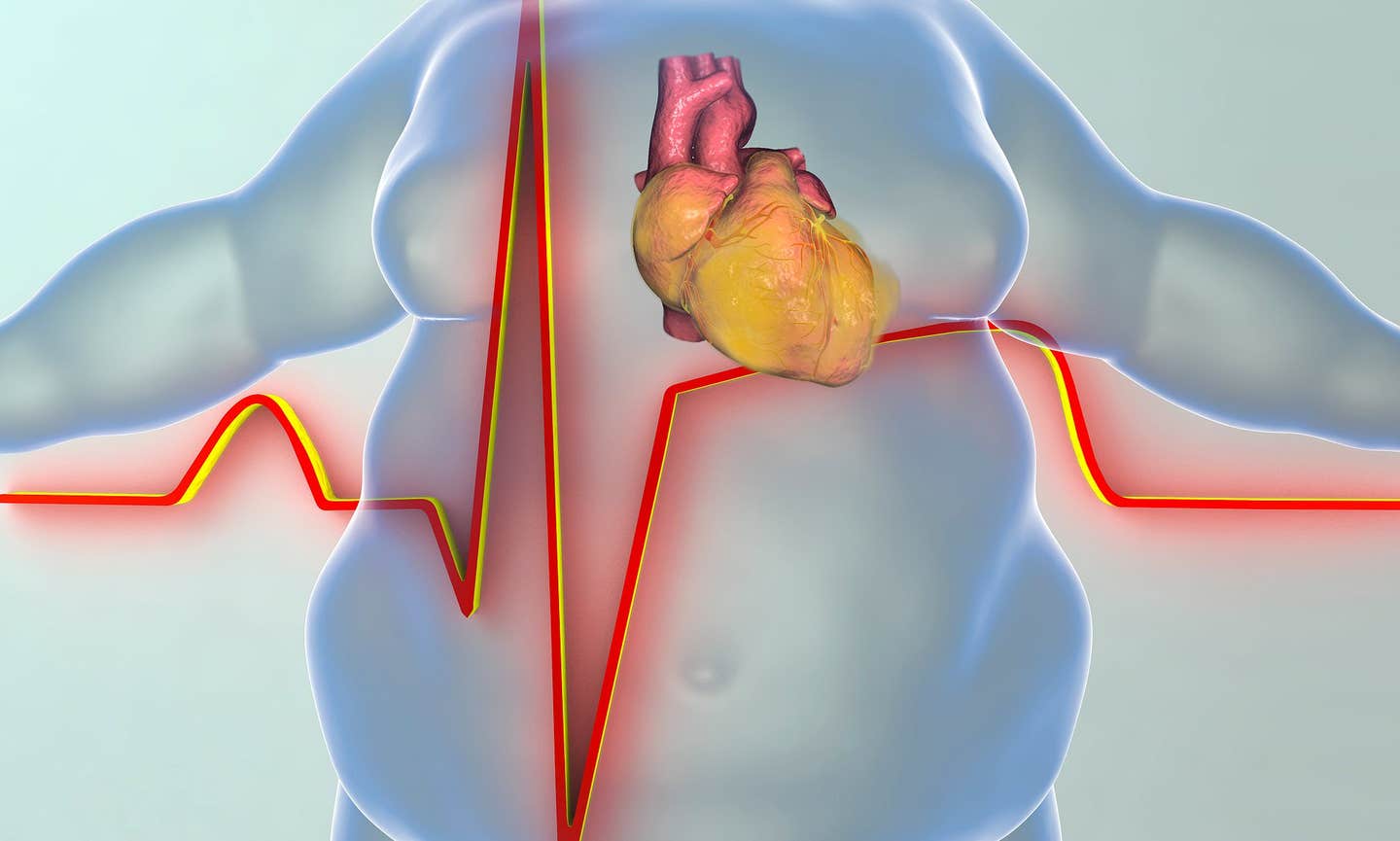
sheds light on how obesity impacts muscle structure in patients with heart failure with preserved ejection fraction (HFpEF). (CREDIT: CC BY-SA 3.0)
A recent study conducted by Johns Hopkins Medicine researchers sheds light on how obesity impacts muscle structure in patients with heart failure with preserved ejection fraction (HFpEF). Published in the journal Nature Cardiovascular Research, this study explores the intricate relationship between obesity and this increasingly common form of heart disease.
Heart failure with preserved ejection fraction, or HFpEF, has become a significant concern worldwide. According to the Journal of Cardiac Failure, HFpEF accounts for over half of all heart failure cases globally. In the United States alone, more than 3.5 million individuals are affected by this condition.
Traditionally, HFpEF was linked to high blood pressure, which often resulted in the heart muscle growing excessively (a condition known as hypertrophy) to counteract the increased pressure. However, over the past two decades, a shift has been observed. HFpEF is now more frequently diagnosed in patients who are severely obese and diabetic, as reported by the Journal of the American College of Cardiology.
Despite its prevalence, effective treatments for HFpEF remain limited. Developing new therapies has been challenging due to the lack of studies examining human heart tissue to pinpoint the exact abnormalities present. Given that hospitalization and mortality rates for HFpEF patients remain alarmingly high—30-40% within five years—it is crucial to deepen our understanding of the disease's underlying causes.
“HFpEF is a complex syndrome, involving abnormalities in many different organs,” explains Dr. David Kass, the study's lead investigator and Professor of Medicine at Johns Hopkins University School of Medicine. Although HFpEF is classified as heart failure due to its similar symptoms to other forms of the condition, the heart's contraction function appears normal.
“We call it heart failure (HF) because its symptoms are similar to those found in patients with hearts that are weak. However, with HFpEF, heart contraction seems fine, yet heart failure symptoms still exist,” says Dr. Kass. This complexity has posed significant challenges in treating HFpEF, as standard heart failure drugs have often been ineffective. However, there have been breakthroughs with drugs initially developed for diabetes and obesity.
Related Stories
One such drug is the SGLT2 inhibitor, which was originally designed to treat diabetes. This drug has shown significant promise in treating HFpEF, not only alleviating symptoms but also reducing long-term hospitalization rates and mortality. Additionally, the weight loss drug GLP1-receptor agonist has been tested and found to improve symptoms in HFpEF patients.
Ongoing studies are investigating whether this drug could also reduce mortality and hospitalizations due to heart failure. These findings highlight the potential of repurposing drugs initially developed for diabetes to address the complexities of HFpEF.
To gain deeper insights into how HFpEF affects heart muscle at the microscopic level, the research team conducted a study involving 25 patients diagnosed with varying degrees of HFpEF linked to diabetes and obesity. The team compared heart muscle tissue from these patients with tissue from 14 organ donors whose hearts were considered healthy. By using an electron microscope, which allows for extremely high magnification, the researchers were able to examine the muscle tissue in extraordinary detail.
“Unlike viewing the heart with a traditional microscope, the electron microscope allows us to magnify the image to 40,000 times its size,” explains Dr. Mariam Meddeb, a cardiovascular disease specialist at Johns Hopkins University School of Medicine who conducted the study. This level of magnification offers a clear view inside the muscle cells, revealing structures like mitochondria, which are the cells' energy producers, and sarcomeres, the units of muscle fibers responsible for generating force.
The study uncovered significant ultrastructural abnormalities in the heart tissue of the most obese patients with HFpEF. These abnormalities included swollen, pale, and disrupted mitochondria, the presence of numerous fat droplets, and tattered sarcomeres. Interestingly, these issues were not linked to whether the patients had diabetes and were less severe in patients with lower levels of obesity.
“These results will help those trying to develop animal models of HFpEF, since they show what one wants to generate at this microscopic level,” notes Dr. Kass. The study also raises a critical question: Could reducing obesity through emerging drug therapies reverse these ultrastructural abnormalities and improve outcomes for HFpEF patients?
This new research represents a significant step forward in understanding HFpEF, particularly in the context of obesity's impact on heart disease. By clarifying the role of obesity in HFpEF, this study provides a valuable target for developing therapies that could improve the lives of millions of HFpEF patients.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.