MIT engineers create a tractor beam to manipulate cells from a distance
MIT’s chip-based optical tweezers offer a compact, sterile way to manipulate cells, advancing biological research without contamination.
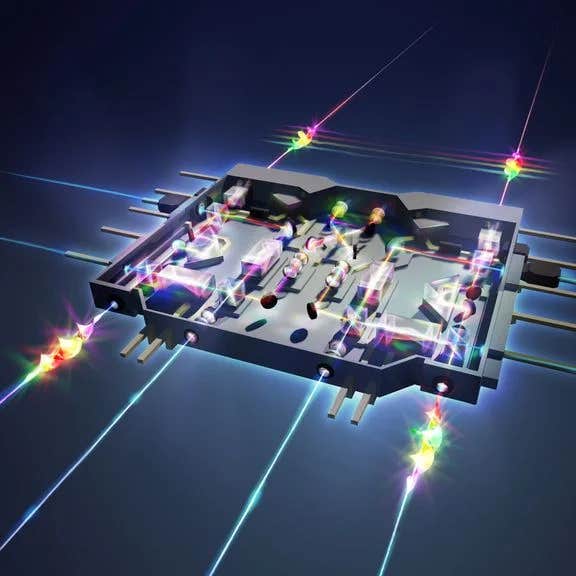
This chip-based “tractor-beam,” which uses an intensely focused beam of light to capture and manipulate biological particles without damaging the cells, could help biologists study the mechanisms of diseases. (CREDIT: Sampson Wilcox, RLE)
Researchers at MIT have developed a groundbreaking chip-based device that could revolutionize how biological experiments are conducted. Using an advanced version of optical tweezers, this device manipulates particles with beams of light, much like the iconic “tractor beam” from Star Wars.
Unlike conventional methods that rely on bulky microscopes, this compact solution is not only mass-manufacturable but also portable and efficient. Its ability to manipulate particles without contaminating the sample offers promising applications for biologists and clinicians studying DNA, classifying cells, and researching disease mechanisms.
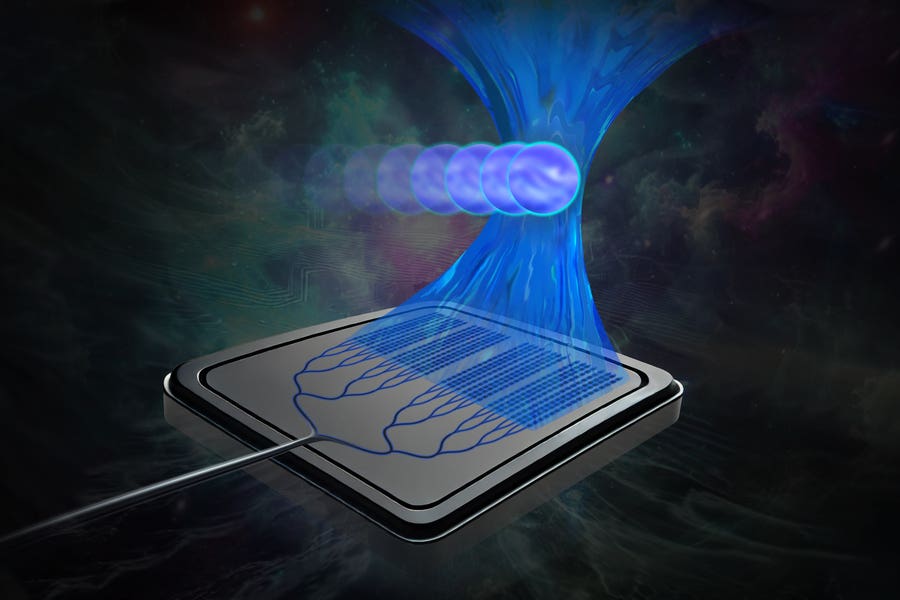
This miniature device, small enough to fit in the palm of your hand, operates through a silicon-photonics chip that generates light to manipulate particles. Unlike existing methods, which require bulky equipment, the light emitted from the chip can penetrate the glass cover slips that shield biological samples. This innovation allows cells to stay within a sterile environment during experiments, preventing contamination and reducing stress on the samples.
Traditional optical tweezers, which are widely used in labs, work by trapping particles with a focused beam of light. These systems typically rely on large microscope setups, limiting their portability and use. However, chip-based optical tweezers offer a compact and scalable alternative.
Still, the challenge has been that similar integrated devices could only trap cells or particles very close to the chip surface. Such close proximity risks contamination and cell stress, making them incompatible with many standard biological experiments.
MIT researchers have found a way around this limitation using a system called an integrated optical phased array. This new technology allows the light beam to trap and manipulate cells more than 100 times further from the chip surface than previously possible, setting a new standard in the field.
As Professor Jelena Notaros, who led the research, explains, “This work opens up new possibilities for chip-based optical tweezers by enabling trapping and tweezing of cells at much larger distances than previously demonstrated.”
Related Stories
The new chip, which was recently detailed in Nature Communications, could eventually transform how scientists study cells. Notaros and her team, including lead author and graduate student Tal Sneh, developed this system with precision in mind, enabling it to work on a much larger scale than prior chip-based optical tweezers.
By manipulating light through the phased array, the researchers could control its focus and movement over millimeter-scale distances, a major improvement compared to earlier technologies.
The process works by utilizing focused beams of light that capture tiny particles and pull them toward the center of the beam. By steering this light, researchers can then move these particles without direct contact. This non-invasive method is key in biological research, where maintaining sterile environments is crucial.
“With silicon photonics, we can take this large, typically lab-scale system and integrate it onto a chip,” says Notaros. This technology presents a cleaner, simpler, and more efficient approach to conducting experiments with delicate biological specimens.
One of the main advantages of this new device is that it avoids the contamination problem plaguing previous methods. Older chip-based systems required the samples to be placed directly on the chip, risking contamination each time an experiment was conducted.
Once contaminated, the chip and the sample would need to be discarded, creating waste and increasing costs. The new system, however, can manipulate samples while they remain sealed under glass cover slips, protecting both the chip and the sample.
The optical phased array technology behind this system uses a series of microscale antennas fabricated on the chip itself. Each antenna emits light, which can be controlled electronically to steer and shape the beam. By focusing the beam tightly, the researchers can create the necessary forces to trap and move microparticles, including biological samples like cancer cells, as demonstrated in their experiments.
Previous phased arrays weren't suitable for generating the kind of tightly focused light beams necessary for biological applications. The MIT team, however, developed specific phase patterns for each antenna to achieve the required focus, overcoming a significant hurdle in applying this technology to biophysics. “No one had created silicon-photonics-based optical tweezers capable of trapping microparticles over a millimeter-scale distance before,” Notaros says, highlighting the leap in capability.
Testing began with polystyrene spheres, a common material for calibrating such devices. Once the researchers succeeded with these spheres, they moved on to cancer cells, provided by collaborator Joel Voldman’s lab. Manipulating these more complex biological particles required the team to overcome several new challenges, including tracking the motion of the cells and adjusting the strength of the light beam to effectively hold them in place.
“There were many unique challenges that came up in the process of applying silicon photonics to biophysics,” explains lead researcher Tal Sneh. Among the issues were determining how to automate some processes and optimizing the system to ensure precision and reliability. Despite these challenges, the team successfully demonstrated that this new chip-based system can trap and manipulate cancer cells, marking a significant achievement in the field.
Looking ahead, the team aims to refine the system to allow for adjustable focal heights, enabling even more versatility in biological experiments. They also plan to explore using the device in more complex applications, such as manipulating multiple biological particles simultaneously at different locations.
By offering a compact, mass-manufacturable solution that can keep biological samples sterile and reduce contamination, this new optical tweezers technology could significantly advance biological research. The ability to manipulate cells from a distance opens the door to new types of experiments that were previously impossible or too cumbersome to perform.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.