MIT develops tiny devices to monitor and heal individual cells – restoring lost brain functions
MIT’s new tiny wearables wrap around neurons to monitor or heal, opening new treatments for brain diseases like multiple sclerosis.
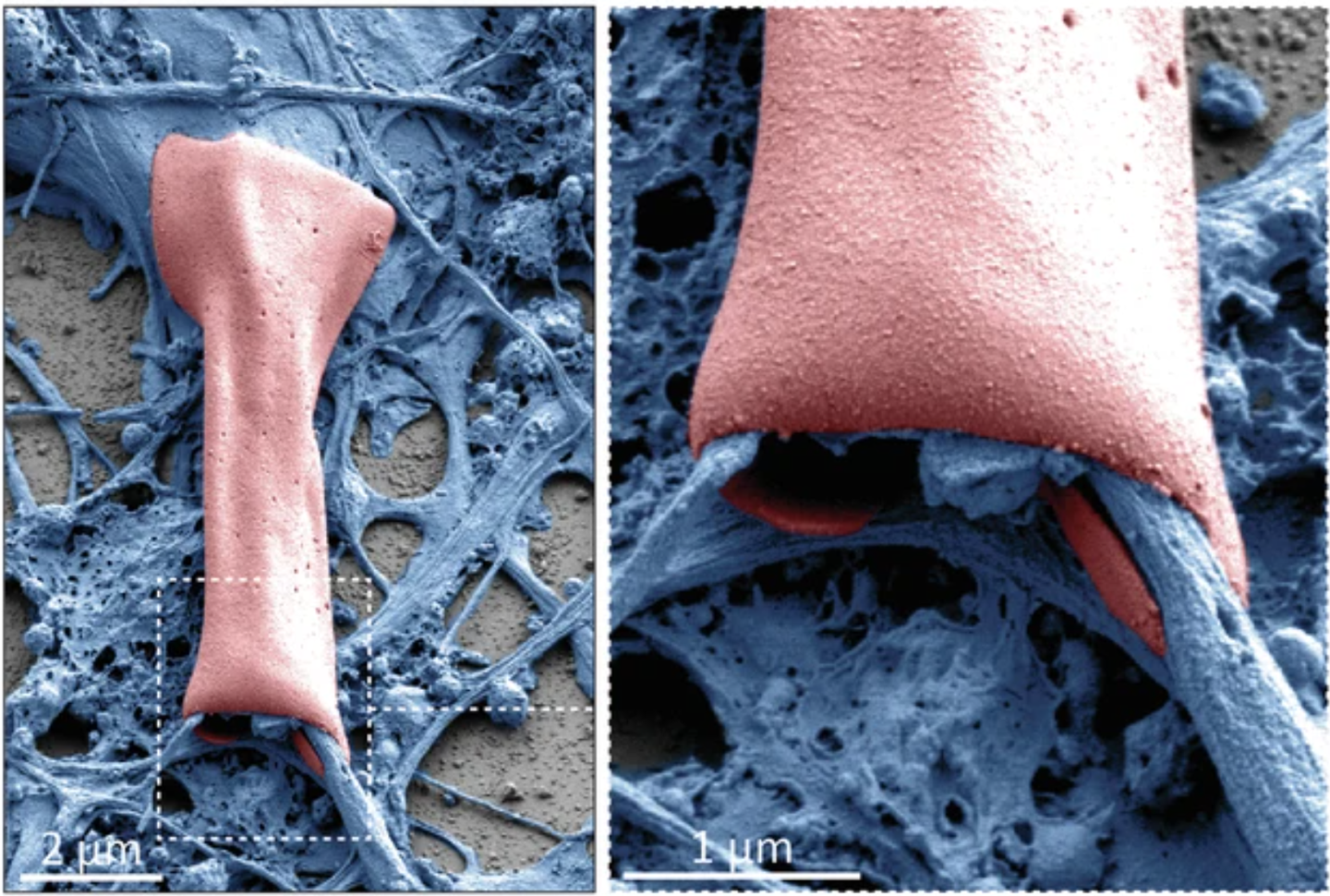
MIT researchers have developed tiny, wireless devices that wrap around neurons. (CREDIT: Nature Communications Chemistry)
Recent advances from MIT researchers are paving the way for unprecedented methods to monitor and potentially heal individual cells, with the focus now on neurons. Traditionally, cells have been seen as basic units of life, but they’re remarkably complex.
In neurons, various activities occur within distinct subcellular compartments, such as axons, dendrites, and the cell body. Understanding and controlling these specialized regions is critical for probing cellular function and treating neurological diseases.
In a groundbreaking development, MIT scientists have created tiny, battery-free devices capable of interacting directly with neurons. These subcellular-sized devices, made from a soft polymer called azobenzene, are designed to wrap around cellular structures like axons and dendrites without damaging them.
By doing so, these devices allow researchers to measure and modulate a neuron’s activity at a highly specific level, which could one day aid in treating diseases like multiple sclerosis (MS).
The team envisions thousands of these devices being injected and wirelessly activated using light. This light-sensitive material transforms upon exposure, rolling into microtubes that can snugly encase the tiny, curved surfaces of neurons. "It is possible to very finely control the diameter of the rolling," explains Deblina Sarkar, senior author and head of MIT’s Nano-Cybernetic Biotrek Lab, who led this research.
To achieve this, the researchers overcame several technical challenges, such as developing a scalable manufacturing process. Rather than a traditional semiconductor clean room, they utilized a novel technique where they deposited a drop of azobenzene onto a dissolvable layer.
By pressing a stamp onto the drop, they molded thousands of devices, each carefully shaped to wrap around neurons’ various curves. This method allows them to bypass costly cleanroom environments and instead rely on simple etching and baking processes.
Related Stories
The devices’ flexibility is key, as they must conform to the complex shapes of brain cells without causing damage. This adaptability has long been an unmet challenge, especially given that neurons and their processes are incredibly delicate.
The thin-film azobenzene sheets, which roll up into microtubes, make it possible for the devices to envelop axons and other neuron components gently. Sarkar emphasizes, “To have intimate interfaces with these cells, the devices must be soft and able to conform to these complex structures.”
Another major application of this technology lies in treating neurodegenerative diseases, specifically those characterized by loss of myelin. Myelin is an insulating layer that wraps around axons, enabling efficient transmission of electrical impulses.
In conditions like multiple sclerosis, this insulation degrades, disrupting communication between neurons. Since azobenzene acts as an insulator, these devices might serve as synthetic myelin, restoring lost functionality in damaged neurons.
According to lead author Marta J. I. Airaghi Leccardi, the devices might eventually form circuits on a microscopic scale, capable of measuring or even modulating individual cells’ functions. "This technology is possible," says Sarkar.
The team demonstrated that integrating the azobenzene-based devices with optoelectrical materials could stimulate cells, and they showed that atomically thin materials can be added without compromising the device’s structural integrity. This opens up possibilities for the devices to become self-contained sensing tools that deliver electrical, optical, or thermal signals to cells.
These devices offer a glimpse into a future where cellular-level monitoring could be used for more than research. The MIT team imagines them as tiny, minimally invasive tools that could one day modulate neurons’ electrical activity, possibly aiding in brain disease treatments. By forming close connections with neurons, the devices require minimal energy to stimulate specific cellular regions.
The research team included Sarkar, Leccardi, MIT postdoc Benoît X. E. Desbiolles, MIT alum Anna Y. Haddad, and graduate students Baju C. Joy and Chen Song. Their work appears in the journal Nature Communications Chemistry.
Future directions for the research include customizing the device surfaces with molecules that target particular cell types. This could lead to devices that detect and treat distinct neuron types or other cell varieties in the body. For instance, functionalizing the devices with specific molecules might enable them to reach and wrap precisely around axons or synapses of interest.
The potential impact of this technology has attracted praise from experts like Flavia Vitale, an associate professor at the University of Pennsylvania. She says, “This work is an exciting step toward new symbiotic neural interfaces acting at the level of the individual axons and synapses.”
She believes that, integrated with nanoscale materials, these devices could one day serve as versatile platforms for delivering various types of signals to neurons in a minimally invasive manner. Although early tests indicate cytocompatibility, extensive testing remains necessary to confirm safety for in-vivo applications.
The technology also has promising applications beyond the nervous system, potentially providing insight into the workings of many other cell types.
Supported by the Swiss National Science Foundation and the U.S. National Institutes of Health Brain Initiative, this research may one day transform how scientists interface with living cells, fostering medical and technological breakthroughs.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joshua Shavit
Science & Technology Writer | AI and Robotics Reporter
Joshua Shavit is a Los Angeles-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a contributor to The Brighter Side of News, he focuses on positive and transformative advancements in AI, technology, physics, engineering, robotics and space science. Joshua is currently working towards a Bachelor of Science in Business Administration at the University of California, Berkeley. He combines his academic background with a talent for storytelling, making complex scientific discoveries engaging and accessible. His work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.



