Lifechanging molecule reverses signs of aging, boosts brain health, and cuts inflammation
By restoring youthful levels of a specific part of the telomerase enzyme, they have significantly reduced signs and symptoms of aging
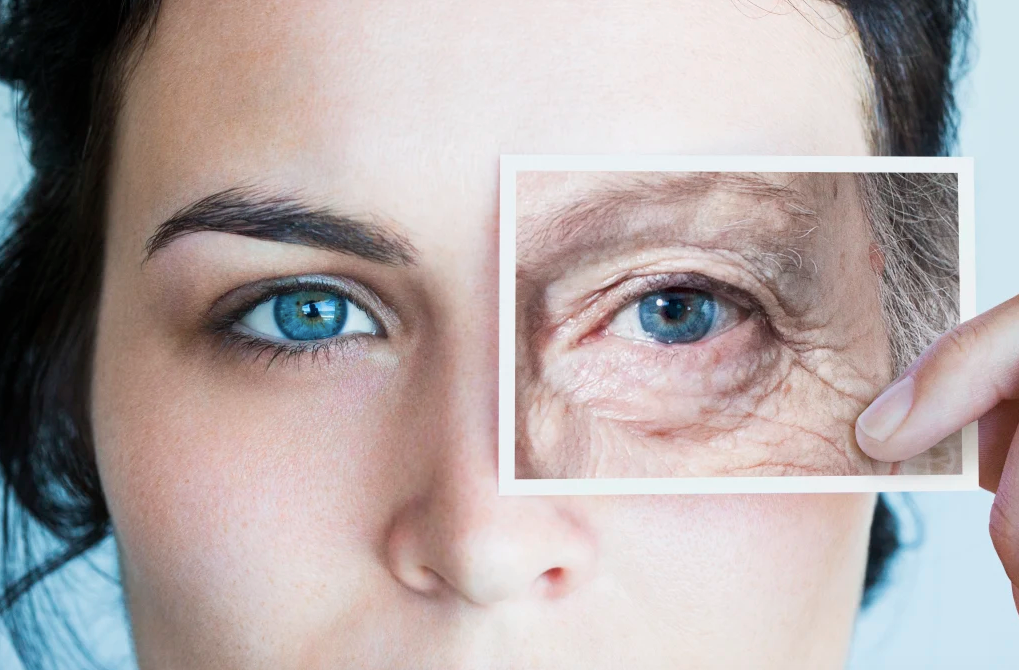
Researchers at The University of Texas MD Anderson Cancer Center have made a groundbreaking discovery in the fight against aging. By restoring youthful levels of a specific part of the telomerase enzyme, they have significantly reduced signs and symptoms of aging in lab models. If these findings hold true in human studies, it could lead to new treatments for age-related diseases like Alzheimer’s, Parkinson’s, heart disease, and cancer.
The study, published in the journal Cell, discovered a small molecule that restores normal levels of telomerase reverse transcriptase (TERT). TERT is typically suppressed as we age. Maintaining TERT levels in older lab models helped reduce cellular aging and tissue inflammation, stimulated new neuron growth with better memory, and enhanced neuromuscular function, leading to increased strength and coordination.
TERT not only extends telomeres, the protective caps at the ends of chromosomes, but also acts as a transcription factor. This means it influences the expression of many genes involved in neurogenesis (the formation of new neurons), learning and memory, cellular aging, and inflammation.
“Epigenetic repression of TERT plays a major role in the cellular decline seen at the onset of aging by regulating genes involved in learning, memory, muscle performance, and inflammation,” said Ronald DePinho, M.D., professor of Cancer Biology and the study's corresponding author. "By pharmacologically restoring youthful TERT levels, we reprogrammed the expression of those genes, resulting in improved cognition and muscle performance while eliminating hallmarks linked to many age-related diseases.”
The Connection Between TERT and Aging
Aging is linked to various epigenetic changes that affect functional and physiological decline. One hallmark of aging is the gradual shortening of telomeres, which maintain chromosome stability. Free radicals can also damage telomeres.
When telomeres become too short or damaged, they trigger a constant DNA damage response, leading to cell senescence. Senescent cells release inflammatory factors that cause tissue damage, promoting aging and cancer.
Related Stories:
Telomerase is the enzyme responsible for synthesizing and extending telomeres. However, its activity decreases over time due to the epigenetic silencing of TERT, especially as we naturally age or develop age-related diseases like Alzheimer’s.
DePinho’s lab previously showed that turning off the TERT gene led to premature aging, which could be reversed by reactivating TERT. They found that certain cells, like neurons and cardiac cells, were rejuvenated without needing to divide and synthesize telomeres. This led them to hypothesize that TERT had other roles beyond telomere synthesis and that overall telomerase levels were crucial in the aging process.
Reversing Aging with a Small Molecule
The researchers, led by DePinho and first author Hong Seok Shim, Ph.D., developed a drug to restore TERT levels. They screened over 650,000 compounds and found a small molecule TERT activating compound (TAC) that epigenetically de-represses the TERT gene, restoring its youthful levels.
In lab models equivalent to humans over 75 years old, six months of TAC treatment led to new neuron formation in the hippocampus, the brain's memory center, and improved performance on cognitive tests. TAC also increased the expression of genes involved in learning, memory, and synaptic biology, consistent with TERT’s role in controlling the activity of transcription factor complexes that regulate diverse genes.
TAC treatment significantly reduced inflammaging, an age-related increase in inflammatory markers linked to multiple diseases, in both blood and tissue samples. It also eliminated senescent cells by repressing the p16 gene, a key factor in cellular aging.
The treatment improved neuromuscular function, coordination, grip strength, and speed in the models, effectively reversing sarcopenia, a condition where muscle mass and performance decline with age. TAC also increased telomere synthesis and reduced DNA damage at telomeres in human cell lines, extending the cells' proliferative potential.
“These preclinical results are encouraging, as TAC is easily absorbed by all tissues, including the central nervous system. Yet further studies are needed to properly assess its safety and activity in long-term treatment strategies,” DePinho said. “However, our deeper understanding of the molecular mechanisms driving the aging process has uncovered viable drug targets, allowing us to explore opportunities to intercept the causes of a variety of major age-related chronic diseases.”
Implications for Age-Related Diseases
If TAC proves to be safe and effective in humans, it could revolutionize how we treat age-related diseases. Alzheimer's, Parkinson's, heart disease, and cancer are all linked to the aging process and could potentially be slowed or even reversed with treatments that restore TERT levels.
Alzheimer's disease, for instance, is characterized by the accumulation of amyloid plaques and tau tangles in the brain, leading to neurodegeneration and cognitive decline. By promoting new neuron formation and reducing inflammation, TAC could help preserve brain function and slow the progression of Alzheimer's.
Similarly, Parkinson's disease involves the loss of dopamine-producing neurons in the brain, leading to motor symptoms like tremors and stiffness. TAC's ability to improve neuromuscular function and reduce senescence could help maintain motor function and delay disease progression.
Heart disease, which is often associated with the buildup of fatty deposits in the arteries, could also benefit from TAC's anti-inflammatory effects. By reducing systemic inflammation and improving cellular health, TAC could help prevent or mitigate the damage caused by heart disease.
Finally, cancer, which is driven by genetic mutations and uncontrolled cell growth, could be impacted by TAC's ability to extend telomeres and reduce DNA damage. By maintaining genomic stability, TAC might reduce the risk of cancerous transformations in aging cells.
The discovery of TAC is a significant step forward in understanding and potentially treating the aging process. While more research is needed to confirm its safety and effectiveness in humans, the preclinical results are promising. By targeting the underlying mechanisms of aging, TAC and similar compounds could open the door to new therapies for a range of age-related diseases, offering hope for healthier and longer lives.
By restoring youthful levels of TERT, scientists have demonstrated a potential method to combat the effects of aging and improve overall health. As this research progresses, it holds the potential to revolutionize how we approach aging and age-related diseases, bringing us closer to a future where age is just a number.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.
Joshua Shavit
Science & Technology Writer | AI and Robotics Reporter
Joshua Shavit is a Los Angeles-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a contributor to The Brighter Side of News, he focuses on positive and transformative advancements in AI, technology, physics, engineering, robotics and space science. Joshua is currently working towards a Bachelor of Science in Business Administration at the University of California, Berkeley. He combines his academic background with a talent for storytelling, making complex scientific discoveries engaging and accessible. His work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.