Lab-grown T cells engineered to live longer and fight cancer more effectively
A new way to grow T cells improves cancer immunotherapy by enhancing their survival and effectiveness against tumors.
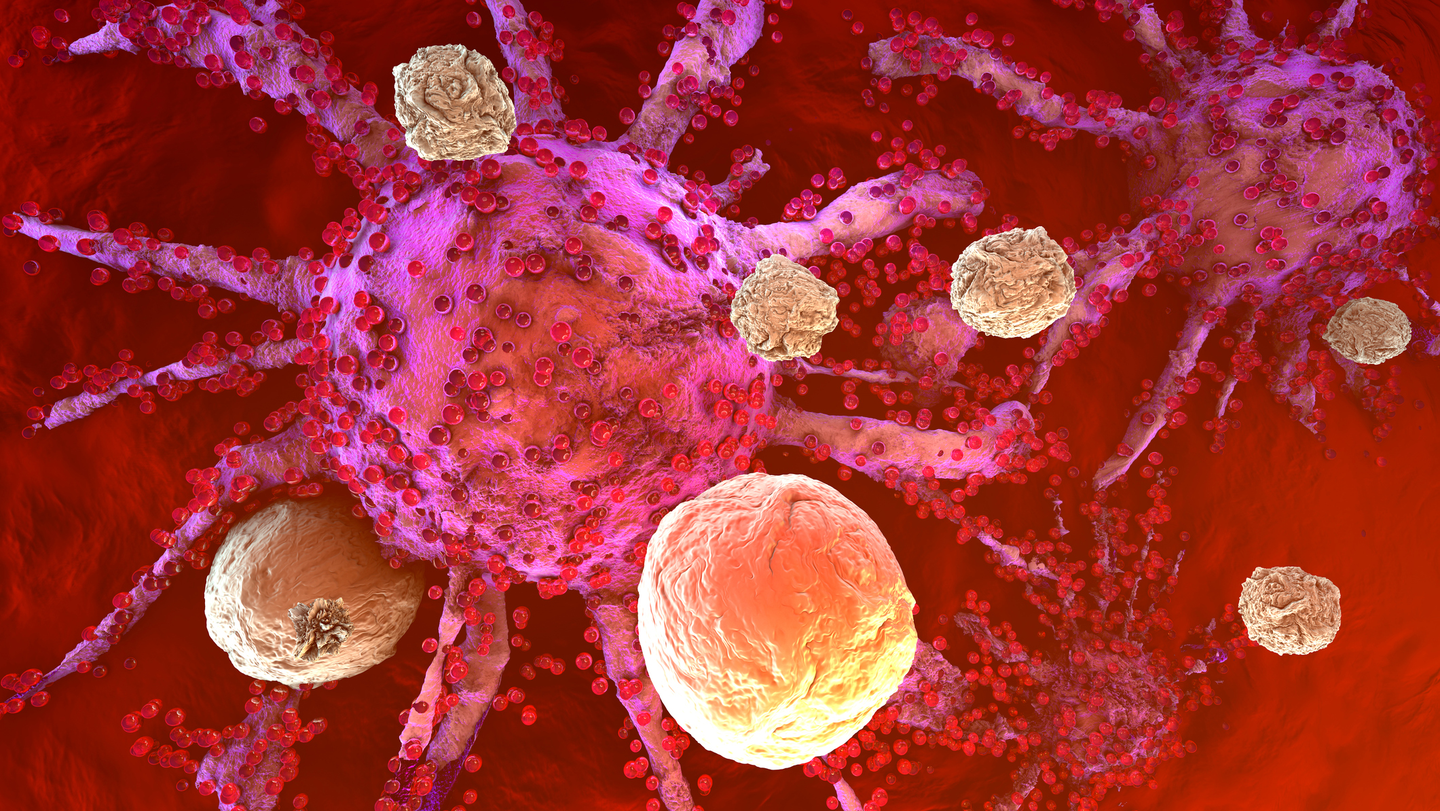
Scientists have developed a new way to grow T cells, making them last longer and fight cancer more effectively. (CREDIT: CC BY-SA 4.0)
Researchers have developed a new method for growing T cells in the lab, allowing them to survive longer and fight cancer more effectively. This advancement, detailed in Cell Metabolism, could significantly improve immunotherapy, a treatment that harnesses the body's immune system to target cancer.
Immunotherapy has transformed cancer treatment, with strategies like immune checkpoint blockade and adoptive cell therapy (ACT) helping the body’s T cells attack tumors. However, these treatments face major hurdles, particularly in solid tumors. While ACT has shown success against blood cancers, solid tumors present metabolic barriers that weaken T cells before they can eliminate cancer cells.
A major challenge in ACT is the tumor microenvironment (TME), which starves T cells of essential nutrients and alters their function. When T cells face continuous stimulation without proper metabolic support, they lose their ability to fight tumors.
Scientists have been working to improve the metabolic resilience of these cells, but the traditional lab methods used to expand T cells may be part of the problem.
A New Approach to T Cell Culturing
T cells are typically grown in the lab with high levels of glucose and other nutrients under the assumption that more is better. However, researchers found that this approach creates cells that are metabolically unprepared for survival inside the body.
Senior author Dr. Greg Delgoffe, a professor at the University of Pittsburgh, explained, “The way we traditionally grow T cells in the lab is horribly inefficient. We make millions of T cells and infuse them into a patient, but most of the cells die.”
To address this, the research team investigated the metabolism of lab-grown T cells and identified a key enzyme, pyruvate dehydrogenase kinase 1 (PDHK1), which plays a role in their high dependence on glucose. When this enzyme was inhibited during cell growth, the T cells became more adaptable to alternative energy sources.
Related Stories
To improve their longevity, researchers added dichloroacetate (DCA) to the cell culture. This compound shifts T cells away from excessive glucose dependence, training them to use energy sources found in the body.
Lead author Andrew Frisch, a graduate student at the University of Pittsburgh, noted, “Cell therapy is a living drug that responds to the environment in the body. But there is a major gap between where we are with these therapies and where we could be because the way we feed these cells in the lab does not prepare them well for surviving in the body.”
Enhanced Survival and Tumor Response
The benefits of the modified T cell culture method became evident in mouse models. When T cells grown with DCA were infused into mice, they survived significantly longer than those cultured with traditional methods.
Nearly a year later, over 5% of the transferred T cells remained in circulation. In contrast, mice given traditionally grown T cells retained almost none within a few weeks.
Mice with melanoma showed improved tumor control and longer survival when treated with the DCA-grown T cells. The benefits extended beyond initial treatment. When these mice were later challenged with new melanoma cells, their immune systems mounted a strong response, suggesting long-term protection.
By shifting how T cells are cultured, researchers may be able to create more effective cell therapies with lasting immunity against cancer.
“By limiting the access to certain foods, we endowed immune cells with the ability to metabolize things that they would normally metabolize in the body, rather than getting them addicted to the sugar that we were feeding them in the lab,” said Delgoffe.
The Future of Cell Therapy
This breakthrough offers hope for more effective immunotherapies. The goal is to create long-lived immune cells capable of patrolling the body indefinitely.
“If we can properly nourish our T cell soldiers in the lab by convincing them to eat the right kind of food, they are better prepared to respond to signals in the body and live much longer,” Delgoffe said. “We might be able to have a soldier on guard forever.”
This research could lead to major improvements in CAR-T and TIL therapies, which already show promise in cancer treatment. By refining the metabolic conditions under which T cells are grown, scientists are moving closer to making cancer immunotherapy more durable and effective.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joshua Shavit
Science & Technology Writer | AI and Robotics Reporter
Joshua Shavit is a Los Angeles-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a contributor to The Brighter Side of News, he focuses on positive and transformative advancements in AI, technology, physics, engineering, robotics and space science. Joshua is currently working towards a Bachelor of Science in Business Administration at the University of California, Berkeley. He combines his academic background with a talent for storytelling, making complex scientific discoveries engaging and accessible. His work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.