Lab-grown neurons developed to behave exactly like natural ones
Lab-grown neurons restructured with microfluidic devices mimic real brain activity, aiding memory and learning research in controlled environments.
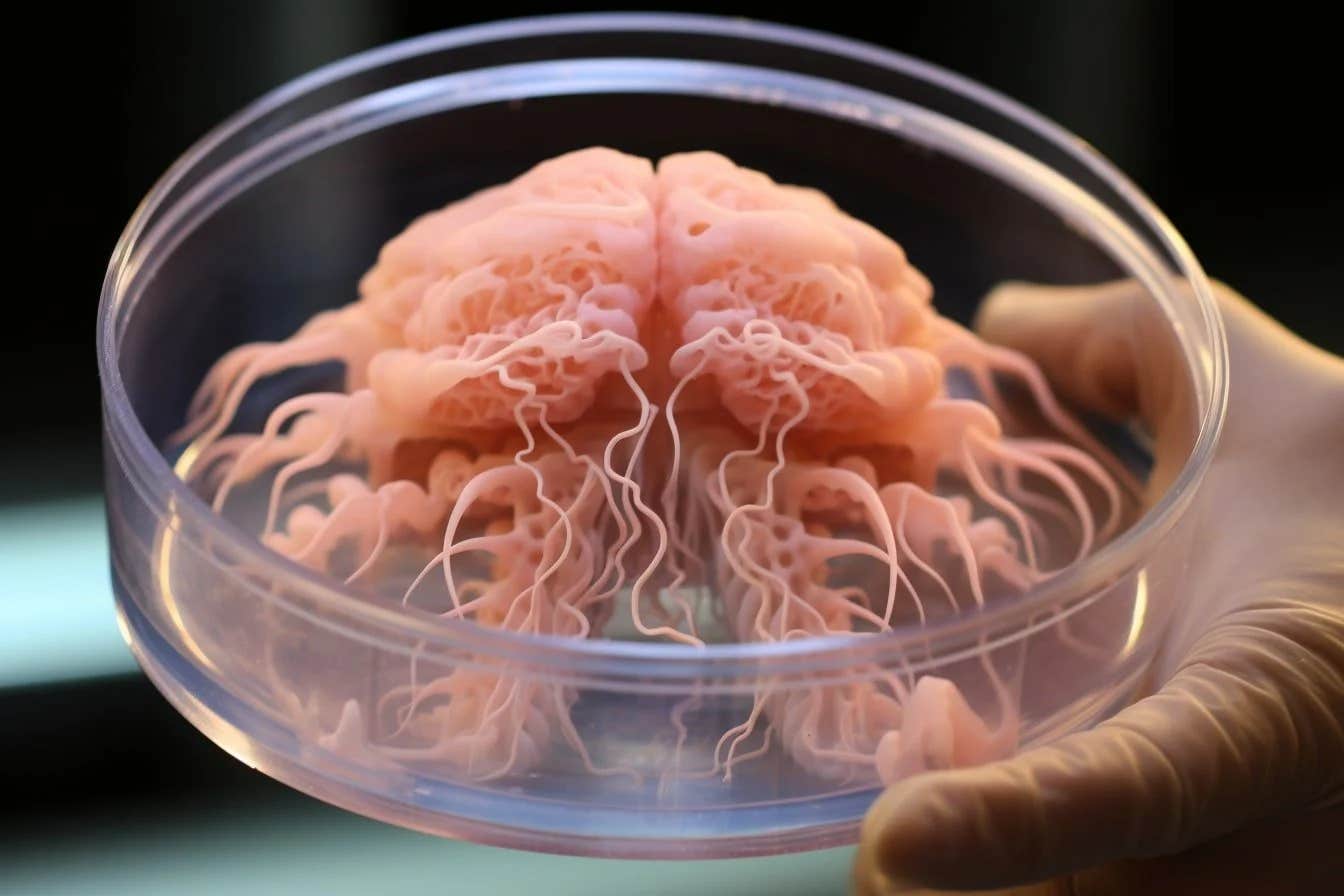
Lab-grown neurons, restructured using microfluidic devices, exhibit diverse and adaptive activity patterns. (CREDIT: CC BY-SA 4.0)
In the intricate world of neuroscience, the phenomenon of neuronal ensembles offers a fascinating glimpse into the building blocks of the brain's information processing. These ensembles are coordinated groups of neurons firing together in specific regions of the brain, including the visual, auditory, and somatosensory cortices. Their activity patterns aren’t random but tied closely to sensory inputs, memory formation, and behavioral responses.
In the visual cortex, for instance, particular neuronal ensembles respond consistently to visual stimulation, forming predictable patterns that persist even during spontaneous neural activity. Similar properties have been observed in the auditory cortex and somatosensory regions, where specific ensembles link directly to pain-related behaviors in mice.
Evidence suggests these groups of neurons are critical for encoding and storing information, underscoring their importance in understanding how brains process and retain data.
Studying neuronal ensembles in living animals offers valuable insights, but the complexity of in-vivo systems presents challenges. To overcome these, researchers often turn to in-vitro models, where neurons are grown in controlled environments.
In standard lab cultures, however, neurons form randomly connected networks that exhibit excessive synchrony. Instead of mimicking the diverse, structured patterns of natural neuronal activity, these networks tend to fire in unison, limiting their usefulness for understanding the nuanced dynamics of brain function.
To address this, scientists have turned to innovative engineering techniques, such as micropatterned substrates, microfluidic devices, and engineered scaffolds. Among these, microfluidic devices have proven particularly effective.
These small chips contain reservoirs and microchannels that guide neurons to form structured connections, enabling researchers to manipulate how neurons grow and interact. By adjusting the size and shape of these microchannels, the strength of connections between neurons can be precisely controlled.
A breakthrough in this field came from a team at Tohoku University, led by Hideaki Yamamoto, who used microfluidic devices to develop lab-grown neuronal networks that closely mimic the connectivity of animal brains. Their findings, published in Advanced Materials Technologies, represent a significant leap forward in creating in-vitro models that behave more like real brain systems.
Related Stories
Yamamoto and his team reconstituted neuronal networks on microfluidic chips, observing activity patterns that mirrored the complexity of natural neural ensembles.
They found that reducing the size of microchannels helped suppress the excessive synchrony often seen in lab-grown networks. Instead of a single dominant ensemble, these refined networks could sustain up to six distinct ensembles, closely resembling the diversity found in a living brain.
The ability to control neuronal connectivity also allowed the researchers to explore how these networks respond to repetitive stimulation. Remarkably, the in-vitro neurons exhibited signs of plasticity, reorganizing their ensembles in response to repeated inputs. This adaptability mirrors the way real brains learn and adapt to new information, making these networks a valuable tool for studying processes like memory formation and learning.
One of the most compelling aspects of this research is its potential to bridge the gap between artificial and biological systems. Lab-grown neurons that mimic natural connectivity could open doors to advanced models capable of simulating specific brain functions, such as memory recall or sensory integration. These models could also enhance our understanding of neurological diseases, providing a controlled platform for testing therapies and interventions.
“Lab-grown neurons allow scientists to explore how learning and memory work in highly controlled conditions,” Yamamoto explains. “There is a demand for these neurons to be as close to the real thing as possible.” By leveraging microfluidic technology, his team has taken a significant step toward meeting this demand.
Beyond their immediate applications, these findings have broader implications for neuroscience and bioengineering. The ability to reconfigure neuronal networks offers a glimpse into the potential of creating artificial systems that replicate the brain's remarkable adaptability. This could lead to breakthroughs in areas ranging from cognitive computing to neural repair technologies.
Despite the progress, challenges remain. While microfluidic devices offer precise control over neuronal growth, replicating the full complexity of brain structures is an ongoing task.
Future research will need to refine these models further, integrating more advanced technologies to simulate the intricate interplay of genetic, chemical, and electrical factors that shape natural neural networks.
The promise of these technologies, however, is undeniable. As researchers continue to refine in-vitro models, the gap between artificial and biological systems narrows, bringing us closer to unlocking the mysteries of the human brain.
From understanding memory formation to developing therapies for neurological disorders, these lab-grown networks hold immense potential for advancing science and medicine.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.