Innovative ‘water battery’ produces safer, more affordable power for smart devices
Their groundbreaking research focuses on improving the voltage of aqueous zinc-ion batteries, which utilize a water-based electrolyte.
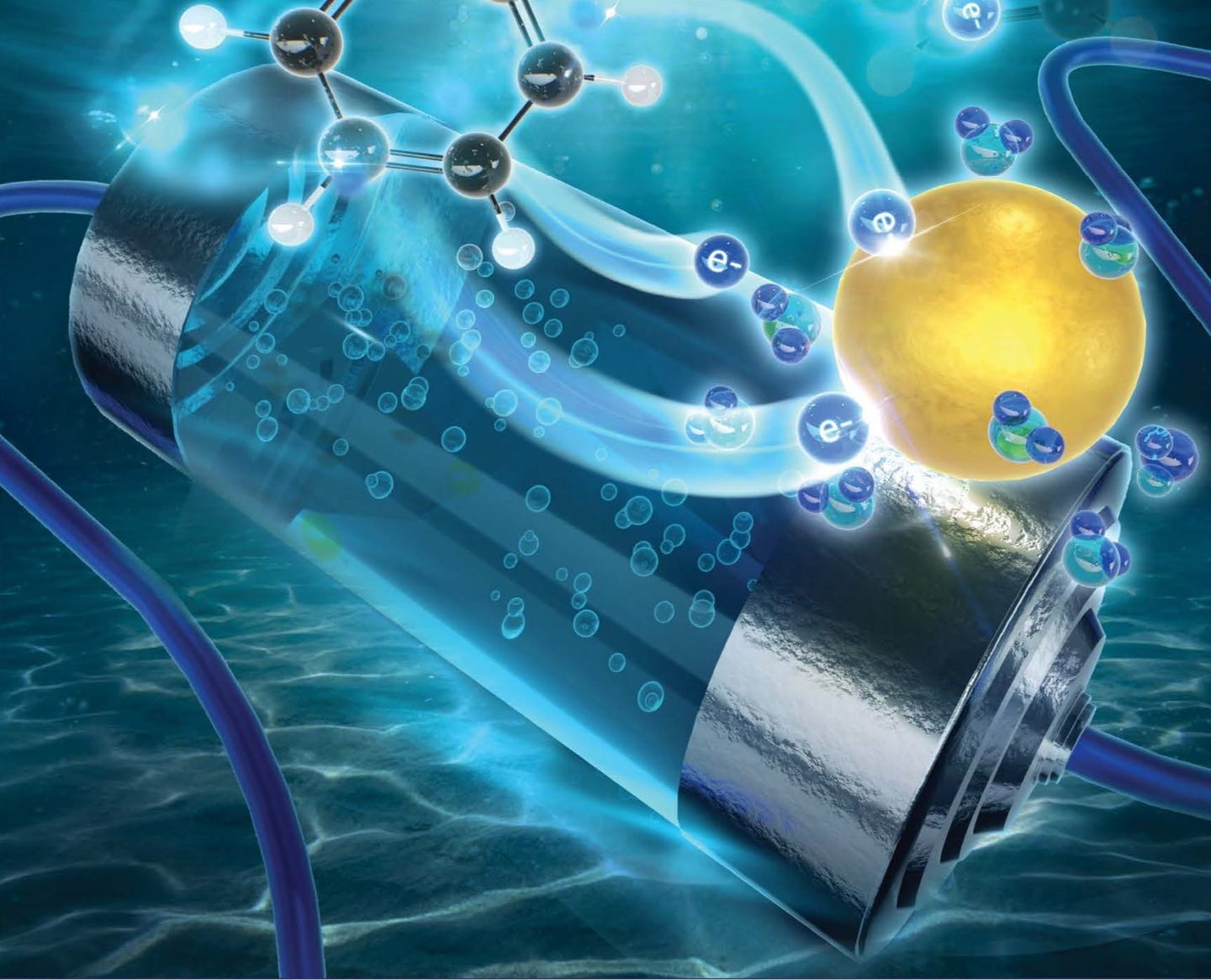
In a significant leap forward for battery technology, a team of international researchers led by Queensland University of Technology (QUT) has developed a safer and more cost-effective type of battery. Their groundbreaking research focuses on improving the voltage of aqueous zinc-ion batteries, which utilize a water-based electrolyte. This advancement could revolutionize the way we power our smart devices.
Batteries are integral to modern life, powering everything from our smartphones to electric vehicles. However, current battery technologies, particularly lithium-ion batteries, come with significant drawbacks. They rely on organic electrolytes, which are not only expensive but also highly flammable, posing safety risks.
"Aqueous batteries have been around for over a century, primarily as non-rechargeable power sources," said Professor Ziqi Sun from QUT's School of Chemistry and Physics and Centre of Materials Science. "Improving their low voltage has been a major challenge, hindering their broader application."
Aqueous zinc-ion batteries stand out as a safer alternative due to their use of water-based electrolytes. Unlike their organic counterparts, these electrolytes are non-flammable and significantly cheaper. However, they have struggled with low voltage, which limits their energy density and overall performance.
"The main obstacle is the low reducing voltage of water, which is about 1.23 volts (V)," explained Fan Zhang, the first author of the research paper. "Exceeding this voltage causes the generation of hydrogen, leading to battery swelling and potential explosions."
The team, including QUT researchers Professor Ziqi Sun, Associate Professor Dongchen Qi, Fan Zhang, Professor Ting Liao, Professor Cheng Yan, and Dr. Aaron Micallef, sought to overcome this hurdle. Their approach drew inspiration from the Marcus Theory of electron transfer, a Nobel Prize-winning concept developed by Rudolph Marcus.
Related Stories:
"In our study, we introduced an organic compound called catechol into the aqueous zinc sulfate electrolyte," Professor Sun said. "This compound alters the electron transfer mechanism from the typical inner sphere transfer to an outer sphere transfer."
In simpler terms, this adjustment allows the catechol compound to stabilize water molecules, preventing the dangerous hydrogen build-up. As a result, the battery can operate at higher voltages safely.
Enhancing Battery Performance
The incorporation of catechol has resulted in a significant improvement in the performance of aqueous zinc-ion batteries. "This new electron transfer mechanism opens up a novel way to design high-voltage aqueous electrolytes," Professor Sun noted. "We've achieved a voltage window that is twice as high, and overall battery performance is improved by 1.5 to three times compared to conventional aqueous electrolytes."
These advancements bring aqueous zinc-ion batteries closer to practical use in various applications. With twice the charge capacity of lithium-ion batteries, zinc-ion batteries promise not only enhanced energy storage but also smaller sizes and faster charging times. Additionally, they boast a much longer recharging cycle life.
The implications of this research are far-reaching. The improvements in voltage and performance make zinc-based aqueous batteries a viable option for industrial production and widespread adoption. "This is a significant step forward for aqueous rechargeable batteries," said Professor Sun. "It marks a big leap towards their use in real-world applications."
The research, published in the prestigious Journal of the American Chemical Society, highlights the potential for aqueous zinc-ion batteries to become a mainstream solution for safer, cheaper, and more efficient energy storage.
This breakthrough is not just a technical achievement but a glimpse into a future where the limitations of current battery technology are overcome, making our everyday devices safer and more reliable.
For more science news stories check out our New Innovations section at The Brighter Side of News.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.