Inhalable powder protects lungs against COVID-19, flu viruses
Researchers developed an inhalable powder that protects lungs and airways from viral invasion by reinforcing the body’s own mucosal layer.
[Feb. 10, 2023: Tracey Peake, North Carolina State University]
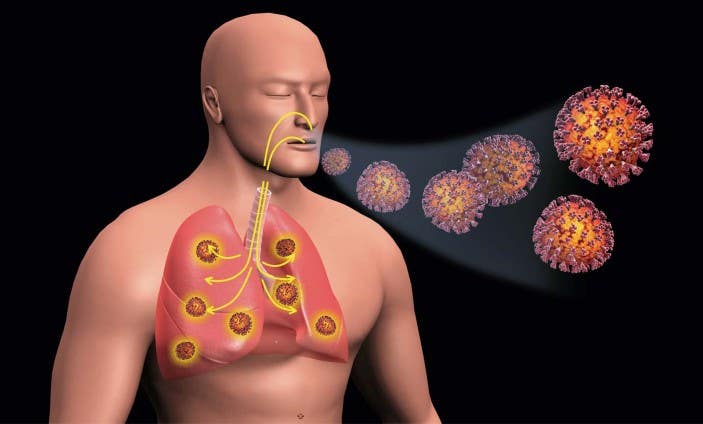
Researchers have developed an inhalable powder that could protect lungs and airways from viral invasion. (CREDIT: Creative Commons)
Researchers have developed an inhalable powder that could protect lungs and airways from viral invasion by reinforcing the body’s own mucosal layer. The powder, called Spherical Hydrogel Inhalation for Enhanced Lung Defense, or SHIELD, reduced infection in both mouse and non-human primate models over a 24-hour period, and can be taken repeatedly without affecting normal lung function.
“The idea behind this work is simple – viruses have to penetrate the mucus in order to reach and infect the cells, so we’ve created an inhalable bioadhesive that combines with your own mucus to prevent viruses from getting to your lung cells,” says Ke Cheng, corresponding author of the paper describing the work. “Mucus is the body’s natural hydrogel barrier; we are just enhancing that barrier.”
Cheng is the Randall B. Terry, Jr. Distinguished Professor in Regenerative Medicine at North Carolina State University’s College of Veterinary Medicine and a professor in the NC State/UNC-Chapel Hill Joint Department of Biomedical Engineering.
The inhalable powder microparticles are composed of gelatin and poly(acrylic acid) grafted with a non-toxic ester. When introduced to a moist environment – such as the respiratory tract and lungs – the microparticles swell and adhere to the mucosal layer, increasing the “stickiness” of the mucus.
Related Stories:
The effects are most potent during the first eight hours after inhalation. SHIELD biodegrades over a 48-hour period, and is completely cleared from the body.
In a mouse model, SHIELD blocked SARS-CoV-2 pseudovirus particles with 75% efficiency four hours after inhalation, which fell to 18% after 24 hours. The researchers found similar results when testing against pneumonia and H1N1 viruses.
In a non-human primate model of both the original and Delta SARS-CoV-2 variants, SHIELD-treated subjects had reduced viral loads – from 50 to 300-fold less than control subjects – and none of the symptoms commonly associated with infection in primates, such as lung inflammation or fibrosis. Since primates do not exhibit the same symptoms of infection as humans, viral load is the standard marker used to determine exposure.
Fabrication and characterization of SHIELD. Schematic showing the concept of the SHIELD method. Dry SHIELD particles (grey spheres) are inhaled and they become swollen (blue spheres) once they are in contact with the mucus layer (pink layer). (CREDIT: Nature Materials)
The researchers also looked at potential toxicity both in vitro and in vivo: 95% of cell cultures exposed to a high concentration (10 mg ml-1) of SHIELD remained healthy, and mice who were given daily doses for two weeks retained normal lung and respiratory function.
“SHIELD is easier and safer to use than other physical barriers or anti-virus chemicals,” Cheng says. “It works like an ‘invisible mask’ for people in situations where masking is difficult, for example during heavy exercise, while eating or drinking, or in close social interactions. People can also use SHIELD on top of physical masking to have better protection.
The morphology of SHIELD after dehydration by 100 % ethanol. The morphology of SHIELD after exposure to 100% ethanol was investigated under optical microscopy and SEM. (CREDIT: Nature Materials)
“But the beauty of SHIELD is that it isn’t necessarily limited to protecting against COVID-19 or flu. We’re looking at whether it could also be used to protect against things like allergens or even air pollution – anything that could potentially harm the lungs.”
The study appears in Nature Materials and is supported by the National Institutes of Health, the American Heart Association, and special funding from the NC State Provost’s Office. The researchers have filed a patent and are working on FDA approval for human use.
Note: Materials provided above by North Carolina State University. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.
Joseph Shavit
Head Science News Writer | Communicating Innovation & Discovery
Based in Los Angeles, Joseph Shavit is an accomplished science journalist, head science news writer and co-founder at The Brighter Side of News, where he translates cutting-edge discoveries into compelling stories for a broad audience. With a strong background spanning science, business, product management, media leadership, and entrepreneurship, Joseph brings a unique perspective to science communication. His expertise allows him to uncover the intersection of technological advancements and market potential, shedding light on how groundbreaking research evolves into transformative products and industries.