Harvesting water from non-humid air – new device helps billions facing chronic shortages
Researchers are improving efficiencies in extracting water from dry air as a potential solution to provide clean drinking water to billions.
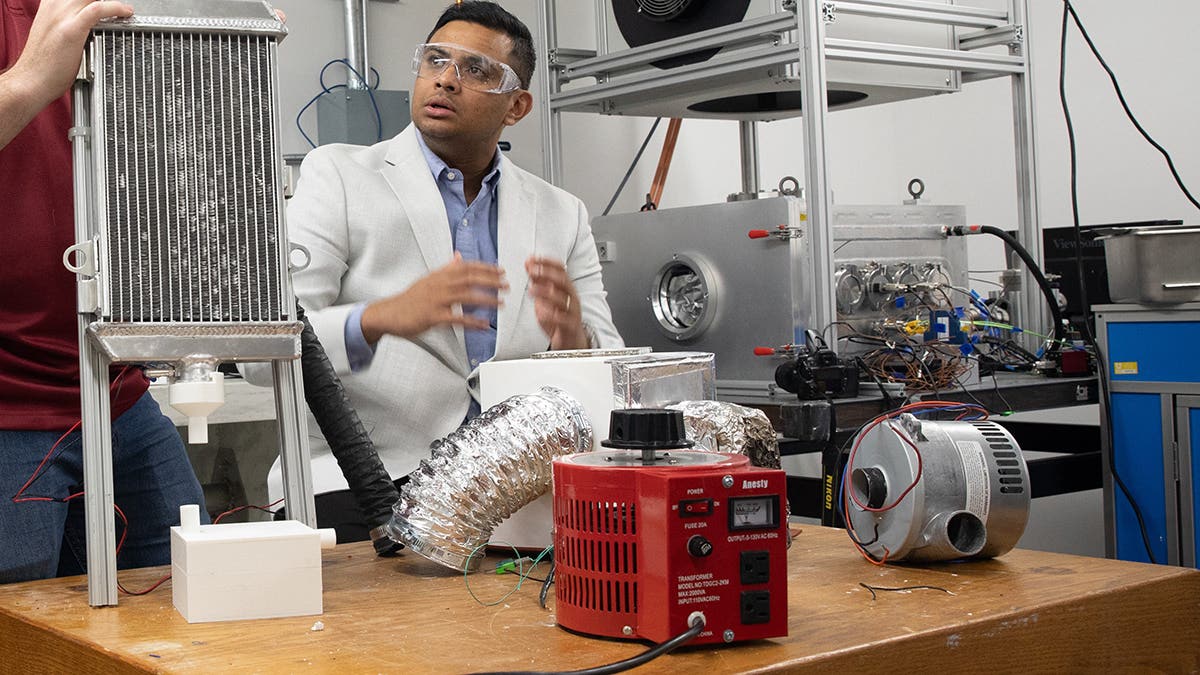
Mechanical engineers Nathan Ortiz, left, and Professor Sameer Rao of the University of Utah describe a device they developed that draws fresh water from the atmosphere. (CREDIT: Dan Hixson, University of Utah)
Earth's atmosphere contains enough water to fill Utah's Great Salt Lake 800 times over. Extracting this moisture is seen as a potential solution to provide clean drinking water to billions facing chronic shortages. However, current atmospheric water harvesting (AWH) technologies face challenges related to size, cost, and efficiency.
Recent research from University of Utah engineers could improve these efficiencies and bring the world closer to tapping the air as a water source in arid regions.
The study introduces a compact, rapid cycling fuel-fired AWH device. This two-step prototype uses adsorbent materials to draw water molecules from non-humid air and then applies heat to release these molecules as liquid water, according to Sameer Rao, senior author of the study and an assistant professor of mechanical engineering.
“Hygroscopic materials intrinsically have an affinity to water. They soak up water wherever you go. One of the best examples is the stuff inside diapers,” said Rao, who has an infant son. “We work with a specific type of hygroscopic material called a metal organic framework.”
Rao compared metal organic frameworks to Lego blocks, which can be rearranged to build various structures. In this case, they are arranged to create a molecule ideal for gas separation. “They can make it specific to adsorb water vapor from the air and nothing else. They're really selective,” he said. Developed with graduate student Nathan Ortiz, the prototype uses aluminum fumarate fashioned into panels that collect water as air is drawn through.
Related Stories
“The water molecules themselves get trapped on the surfaces of our material, and that's a reversible process. Instead of becoming ingrained into the material itself, it sits on the walls,” Ortiz explained. “What's special about these absorbent materials is they have an immense amount of internal surface area. There are many sites for water molecules to get stuck.”
Just a gram of this material holds as much surface area as two football fields, according to Rao. This allows a small amount of material to capture a large amount of water. “All of this surface area is at the molecular scale,” he said. “And that's great for us because we want to trap water vapor onto that surface area within the pores of this material.”
Funding for the research came from the DEVCOM Soldier Center, a Department of Defense program that supports Army modernization. The Army is interested in the project to keep soldiers hydrated in remote areas with few water sources.
“We specifically looked at this for defense applications so that soldiers have a small, compact water generation unit and don’t need to carry a large canteen filled with water,” Rao said. “This would literally produce water on demand.”
Rao and Ortiz have filed for a preliminary patent based on the technology, which also addresses civilian needs. “As we were designing the system, we also considered the broader water problem. It's not just a defense issue; it's very much a civilian issue,” Rao said. “We think in terms of household water consumption for drinking water per day. That's about 15 to 20 liters per day.”
In this proof of concept, the prototype achieved its target of producing 5 liters of water per day per kilogram of adsorbent material. In three days, this device would outperform packing water, according to Ortiz.
In the device’s second step, water is precipitated into liquid by applying heat using a standard Army camping stove. This works due to the exothermic nature of its water-collecting process. “As it collects water, it's releasing little bits of heat. To reverse that, we add heat,” Ortiz said. “We just put a flame right under here, anything to get this temperature up. As we increase the temperature, we rapidly release the water molecules. Once we have a really humid airstream, condensation at ambient temperature becomes much easier.”
Many nascent technologies exist for atmospheric water harvesting, which is easier when the air is humid, but none have resulted in practical equipment for arid environments. Ortiz believes his device can be the first, mainly because it is powered with energy-dense fuel like the white gasoline used in camping stoves.
The team decided against using photovoltaics. “If you're reliant on solar panels, you're limited to daytime operation or you need batteries, which is just more weight. You keep stacking challenges. It just takes up so much space,” Ortiz said. “This technology is superior in arid conditions, while refrigeration is best in high humidity.”
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joshua Shavit
Science & Technology Writer | AI and Robotics Reporter
Joshua Shavit is a Los Angeles-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a contributor to The Brighter Side of News, he focuses on positive and transformative advancements in AI, technology, physics, engineering, robotics and space science. Joshua is currently working towards a Bachelor of Science in Business Administration at the University of California, Berkeley. He combines his academic background with a talent for storytelling, making complex scientific discoveries engaging and accessible. His work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.



