Groundbreaking synthetic molecule found highly effective against drug-resistant bacteria
A groundbreaking new antibiotic could combat the growing threat of drug-resistant bacteria, which poses a major challenge to public health.
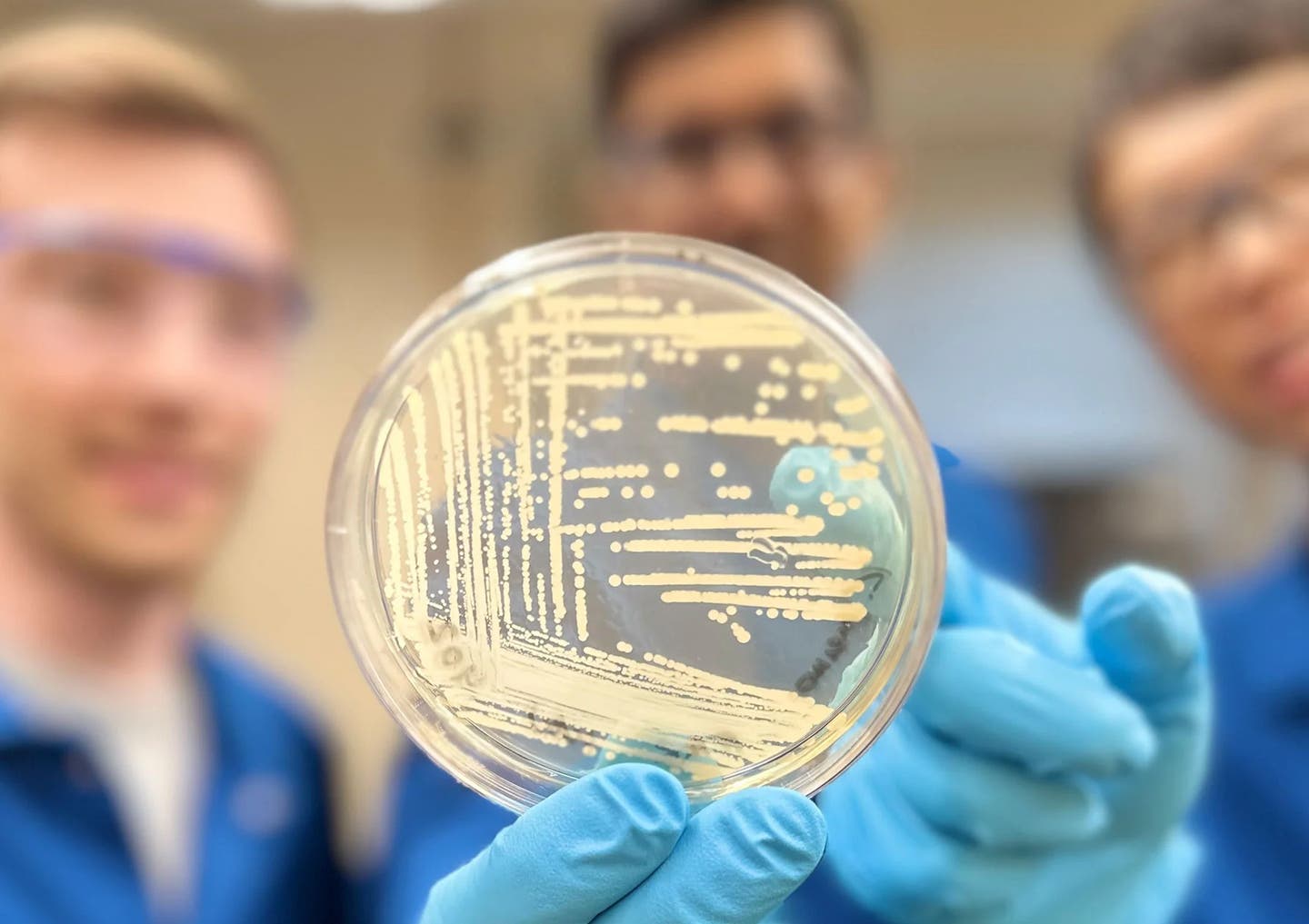
A groundbreaking new antibiotic could combat the growing threat of drug-resistant bacteria, which poses a major challenge to public health. An agar plate containing colonies of Klebsiella pneumoniae bacteria, one of the drug-resistant strains the synthetic compound cresomycin has shown to combat. (CREDIT: Myers Research Group)
Harvard researchers have developed a groundbreaking new antibiotic that could combat the growing threat of drug-resistant bacteria, which pose a significant challenge to public health worldwide.
Led by Andrew Myers, a professor of Chemistry and Chemical Biology at Harvard University, the team unveiled their creation, cresomycin, in a recent publication in the journal Science.
This synthetic compound has demonstrated remarkable effectiveness against various strains of bacteria that have developed resistance to conventional antibiotics, including Staphylococcus aureus and Pseudomonas aeruginosa.
Overview and close-up of cresomycin bound to the bacterial ribosome of Thermus thermophilus. (CREDIT: Yury Polikanov/University of Illinois Chicago)
In the words of Myers himself, "While we don't yet know whether cresomycin and drugs like it are safe and effective in humans, our results show significantly improved inhibitory activity against a long list of pathogenic bacterial strains that kill more than a million people every year, compared with clinically approved antibiotics."
The key to cresomycin's success lies in its enhanced ability to bind to bacterial ribosomes, the cellular structures responsible for protein synthesis. Many existing antibiotics disrupt ribosomal function, but some bacteria have evolved mechanisms to resist these drugs.
Cresomycin, however, circumvents these defenses by adopting a unique structure that tightly grips the ribosome, making it difficult for bacteria to evade its effects.
Related Stories
One of the notable features of cresomycin is its fully synthetic nature, a departure from traditional antibiotics like clindamycin, which are often derived from natural compounds.
This synthetic approach allows for the creation of molecules with novel chemical structures and properties that cannot be achieved through conventional means.
Ben Tresco, a graduate student involved in the research, explains, "The bacterial ribosome is nature's preferred target for antibacterial agents, and these agents are the source of inspiration for our program. By leveraging the power of organic synthesis, we are limited almost only by our imagination when designing new antibiotics."
Comparison of electron density maps of CRM in complex with wild-type, Cfr-, and Erm-modified T. thermophilus 70S ribosomes. (CREDIT: Journal Science)
To overcome the challenge of bacterial resistance, the researchers employed a strategy called component-based synthesis, pioneered in the Myers lab. This method involves constructing molecular components separately before assembling them into the final compound, akin to building sections of a complex LEGO set before putting them together.
This modular approach accelerates the drug discovery process, allowing for the rapid creation and testing of multiple candidate molecules.
Structure of CRM in complex with the wild-type T. thermophilus 70S ribosome. Overview of the CRM-binding site in the 70S ribosome, viewed as a cross-cut section through the nascent peptide exit tunnel (NPET). (CREDIT: Journal Science)
The significance of their work is underscored by the pressing need for effective antibiotics in modern medicine. As Kelvin Wu, another member of the research team, points out, "Antibiotics form the foundation on which modern medicine is built. Without antibiotics, many cutting-edge medical procedures like surgeries, cancer treatments, and organ transplants cannot be done."
The project received critical support from various sources, including a $1.2 million grant from the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), a Boston University-based nonprofit dedicated to advancing antibacterial research and development. Additional funding and support were provided by Harvard's Blavatnik Biomedical Accelerator and the Office of Technology Development, which helped protect and advance the innovations of the Myers Research Group.
Comparisons of m2 m8A2503 positions in Cfr-modified 70S ribosomes, with and without CRM bound. (A, B) Superposition of the structures of Cfr-modified 70S ribosomes, both containing the Cfr-methylated m2 m8 A2503 nucleobase, in the presence (dark blue) and absence (blue) of CRM (green). (CREDIT: Journal Science)
Curtis Keith, chief scientific officer of the Harvard accelerator, emphasizes the importance of such support in driving innovation in antibiotic research. "Funding and other support from groups like the Blavatnik Biomedical Accelerator and CARB-X are essential for the discovery and development of new antibiotics.
These innovations from the Myers Research Group have the potential to yield new drugs that will one day meet a global health need."
With its potent effectiveness and innovative design, this new antibiotic offers hope in addressing one of the most pressing challenges in modern medicine.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.
Joshua Shavit
Science & Technology Writer | AI and Robotics Reporter
Joshua Shavit is a Los Angeles-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a contributor to The Brighter Side of News, he focuses on positive and transformative advancements in AI, technology, physics, engineering, robotics and space science. Joshua is currently working towards a Bachelor of Science in Business Administration at the University of California, Berkeley. He combines his academic background with a talent for storytelling, making complex scientific discoveries engaging and accessible. His work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.



