Game-changing antibody could prevent organ rejection, study finds
Revolutionary discovery opens doors for the potential transformation of transplant medicine and is a beacon of hope for countless patients
[Sept. 4, 2023: Staff Writer, The Brighter Side of News]
This revolutionary discovery opens doors for the potential transformation of transplant medicine and is a beacon of hope for countless patients in need of organ transplants. (CREDIT: Creative Commons)
Duke Health researchers have announced a breakthrough in transplant medicine, revealing that a man-made antibody was successful in preventing organ rejection in primates that underwent kidney transplants. This revolutionary discovery opens doors for the potential transformation of transplant medicine and is a beacon of hope for countless patients in need of organ transplants.
The groundbreaking findings were published online in the esteemed journal, Science Translational Medicine.
While organ transplantation has undoubtedly saved numerous lives, one of the persistent challenges faced by the medical community is the body's instinctual rejection of the new organ. To counteract this, doctors rely on immunosuppressive drugs. Though effective, these medications are a double-edged sword.
"Current medications to prevent organ rejection are good overall, but they have a lot of side effects,” remarked Dr. Imran J. Anwar, M.D., the lead author of the study and a surgical research fellow in Duke’s Department of Surgery. "These therapies suppress the immune system, putting patients at risk of infections and organ damage, and many cause non-immune complications such as diabetes and high blood pressure."
Related Stories:
Thus, the medical and scientific communities have been on a quest, spanning decades, for a safer, less toxic solution. "The push over the last decades has been to develop new, less toxic drugs," added Dr. Anwar. "We are hopeful this antibody moves us closer to that goal."
The said antibody, named AT-1501, is the star of this recent research. The Duke team, which included co-senior author Dr. Allan Kirk, M.D., Ph.D., chair of the Department of Surgery, engineered AT-1501 with the aim to tackle a critical issue faced by its predecessor: the risk of blood clots.
During their study, primates that underwent kidney transplantation were treated with AT-1501. The outcomes were overwhelmingly positive. The antibody managed to prevent organ rejection without the need for any additional immunosuppressive drugs and, importantly, without promoting blood clots.
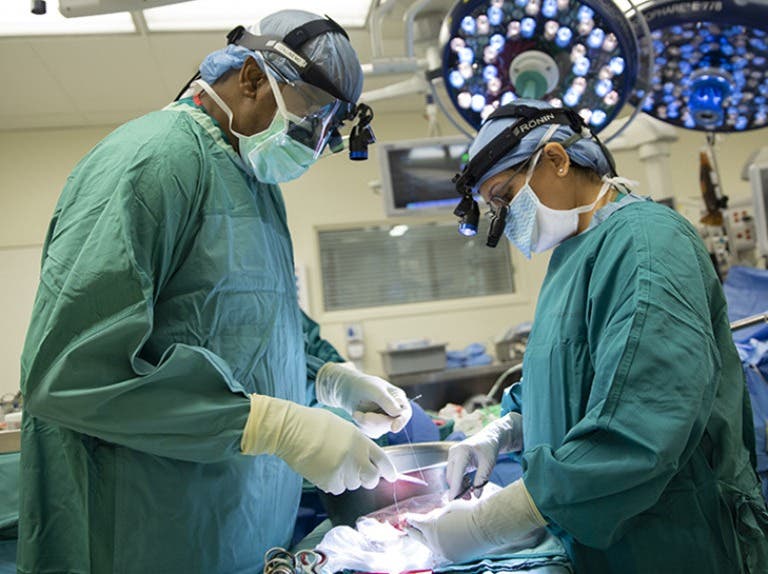
AT-1501 was tested in both a cynomolgus macaque model of intrahepatic islet allotransplantation and a rhesus macaque model of kidney allotransplantation. (CREDIT: Science)
But the benefits of AT-1501 were not limited to just kidney transplants. When tested on animals that had undergone islet transplantation, AT-1501 did not lead to a consistent rejection control on its own. However, when used in combination with existing immunosuppressive drugs, it proved to be effective.
This combination therapy resulted in consistent islet graft survival and, notably, no associated weight loss or infections — common complications associated with such procedures. This segment of the research was carried out in collaboration with Dr. Norma Kenyon, Ph.D., co-senior author and professor at the University of Miami.
AT-1501 monotherapy led to long-term graft survival in both islet and kidney transplant models, confirming its immunosuppressive potential. (CREDIT: Science)
Dr. Allan Kirk celebrated the outcomes, stating, “These data support AT-1501 as a safe and effective agent to promote both islet and kidney transplant survival and function and allow us to advance into clinical trials right away.” He also added a historic perspective: "This less toxic approach has been pursued for over 20 years, and I think we are finally at a turning point. This could be a great advance for people in need of organ transplants."
This research, while still in its nascent stages, holds vast promise. The potential to shift from the current medications — riddled with side effects and complications — to a more efficient and less toxic solution like AT-1501 could change the landscape of organ transplantation.
AT-1501–based regimens after islet transplant resulted in higher C-peptide, greater appetite leading to weight gain, and reduced occurrence of cytomegalovirus reactivation compared with conventional immunosuppression. (CREDIT: Science)
As clinical trials begin, the world watches with bated breath, hopeful that this antibody could pave the way for a new era in transplant medicine, enhancing the quality of life for patients and increasing the success rates of organ transplants.
For those who have waited years on transplant lists and for families who have watched their loved ones grapple with the after-effects of transplantation, this might just be the news they've been waiting for. Only time, and rigorous scientific examination, will tell if AT-1501 is the game-changer it promises to be.
For more science and technology stories check out our New Discoveries section at The Brighter Side of News.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get the Brighter Side of News' newsletter.
Joseph Shavit
Head Science News Writer | Communicating Innovation & Discovery
Based in Los Angeles, Joseph Shavit is an accomplished science journalist, head science news writer and co-founder at The Brighter Side of News, where he translates cutting-edge discoveries into compelling stories for a broad audience. With a strong background spanning science, business, product management, media leadership, and entrepreneurship, Joseph brings a unique perspective to science communication. His expertise allows him to uncover the intersection of technological advancements and market potential, shedding light on how groundbreaking research evolves into transformative products and industries.