Cutting-edge AI spots cancer and viruses in cells before symptoms appear
New AI tool detects cancer and viruses inside cells before symptoms appear, paving the way for improved diagnostics and personalized treatment
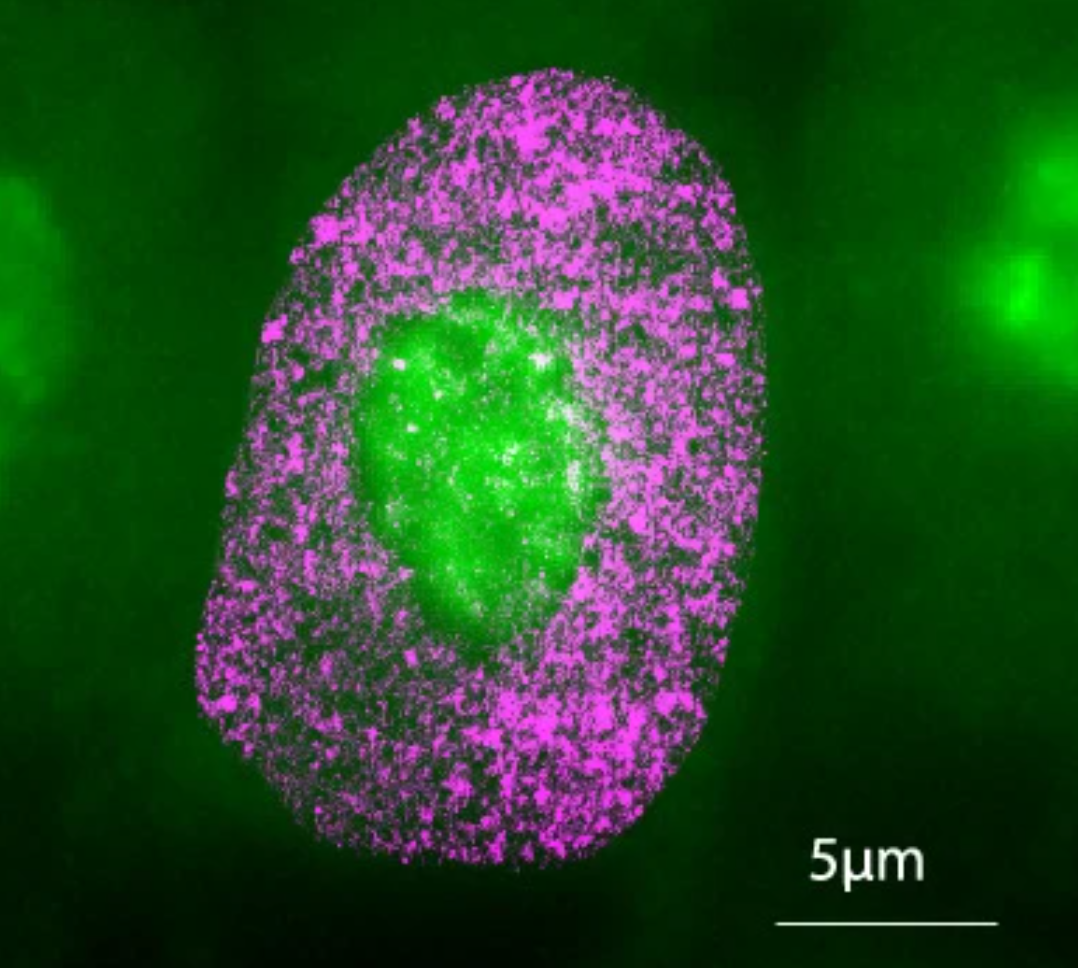
AINU can detect cellular changes as small as 20 nm—5,000 times smaller than the width of a human hair. (CREDIT: Nature Machine Intelligence)
A collaborative team of researchers from institutions like the Centre for Genomic Regulation (CRG), University of the Basque Country (UPV/EHU), Donostia International Physics Center (DIPC), and Fundación Biofisica Bizkaia (FBB) has made significant strides in disease diagnosis through artificial intelligence (AI).
Their recent study, published in Nature Machine Intelligence, reveals a new AI tool, AINU (AI of the NUcleus), capable of distinguishing cancer cells from normal ones and detecting the early stages of viral infections. This advancement could lead to better diagnostic techniques and new disease monitoring strategies, bringing improvements to healthcare.
AINU operates by scanning high-resolution images of cells, obtained using a specialized microscopy method called STORM (Stochastic Optical Reconstruction Microscopy). Unlike regular microscopes, STORM captures finer details of cellular structures at a nanoscale level. To put this scale into perspective, one nanometre (nm) equals one-billionth of a meter, and a human hair is about 100,000 nm wide.
AINU can detect cellular changes as small as 20 nm—5,000 times smaller than the width of a human hair. Such minuscule alterations, often too subtle for traditional methods, become identifiable through the AI’s advanced imaging analysis.
Pia Cosma, a co-corresponding author and ICREA Research Professor at the CRG, explains, “The resolution of these images is powerful enough for our AI to recognize specific patterns and differences with remarkable accuracy, including changes in how DNA is arranged inside cells. This ability could one day give doctors valuable time to monitor diseases, personalize treatments, and improve patient outcomes.”
The AI model, AINU, belongs to a category of convolutional neural networks, an AI architecture particularly suited to processing visual data like images. Such networks are commonly seen in tools like facial recognition software or systems that enable self-driving cars to identify objects on the road. In the medical field, similar AI models assist doctors by analyzing scans such as mammograms and MRIs, identifying signs of disease that might be missed by the human eye.
Related Stories
AINU functions by detecting and analyzing minute structures within cells, focusing on the nucleus. The researchers trained the AI by feeding it nanoscale-resolution images of various cell types and states. This allowed the model to learn how the components of the nucleus are distributed and organized in three dimensions, which it then used to classify cells based on the nuclear characteristics observed.
For example, cancer cells often display distinct changes in their nuclear structure, such as altered DNA organization or enzyme distribution. After training, AINU was able to scan new cell images and accurately determine whether they were cancerous or normal based solely on these features.
Its precision extends beyond cancer detection—AINU also identifies changes as early as one hour after a cell is infected by the herpes simplex virus type-1. These early detection capabilities arise from the AI’s ability to recognize slight variations in how tightly DNA is packed, a hallmark of viral activity altering cellular structure.
According to Ignacio Arganda-Carreras, co-corresponding author and Ikerbasque Research Associate at UPV/EHU, “Our method can detect cells infected by a virus very soon after the infection starts. Normally, it takes time for doctors to spot an infection because they rely on visible symptoms or larger changes in the body.
But with AINU, we can see tiny changes in the cell’s nucleus right away.” He adds that such rapid detection could help researchers better understand how viruses interact with cells immediately after infection, aiding in the development of more effective treatments and vaccines.
Limei Zhong, co-first author of the study and researcher at Guangdong Provincial People's Hospital, shares a vision for clinical use, saying, “AINU could be used in hospitals and clinics to quickly diagnose infections from a simple blood or tissue sample, making the process faster and more accurate.”
However, the technology faces challenges before it can be implemented in clinical settings. STORM imaging requires specialized equipment found primarily in research labs, making it difficult to bring this technology to more routine medical environments.
Additionally, current imaging techniques used in STORM only allow for analyzing a few cells at a time. For the tool to be effective in clinical diagnostics, doctors would need to process larger numbers of cells quickly and efficiently.
Despite these hurdles, Cosma is optimistic. “There are many rapid advances in STORM imaging which mean that microscopes may soon be available in smaller or less specialized labs, and eventually, even in the clinic. The limitations of accessibility and throughput are more tractable problems than we previously thought, and we hope to carry out preclinical experiments soon.”
While clinical applications may still be years away, the researchers expect AINU to significantly impact scientific research in the short term. The AI can identify pluripotent stem cells—those that can develop into any type of cell in the body—with high precision. Pluripotent cells are vital to therapies aimed at repairing or replacing damaged tissues.
Davide Carnevali, first author of the research and a CRG researcher, highlights the implications for stem cell research, saying, “Current methods to detect high-quality stem cells rely on animal testing. However, all our AI model needs to work is a sample stained with specific markers that highlight key nuclear features. As well as being easier and faster, this can accelerate stem cell research while contributing to the shift in reducing animal use in science.”
AINU represents a leap forward in cellular analysis, offering potential advancements in diagnosing diseases and improving treatment strategies. Though there is still work to be done, the promise of this AI tool suggests a future where early detection of diseases, personalized treatments, and efficient stem cell therapies become more accessible and precise.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.



