CRISPR transforms ordinary fat cells into cancer killing machines
Scientists use CRISPR to turn fat cells into cancer-fighting machines, cutting off tumors’ nutrient supply and halting growth.
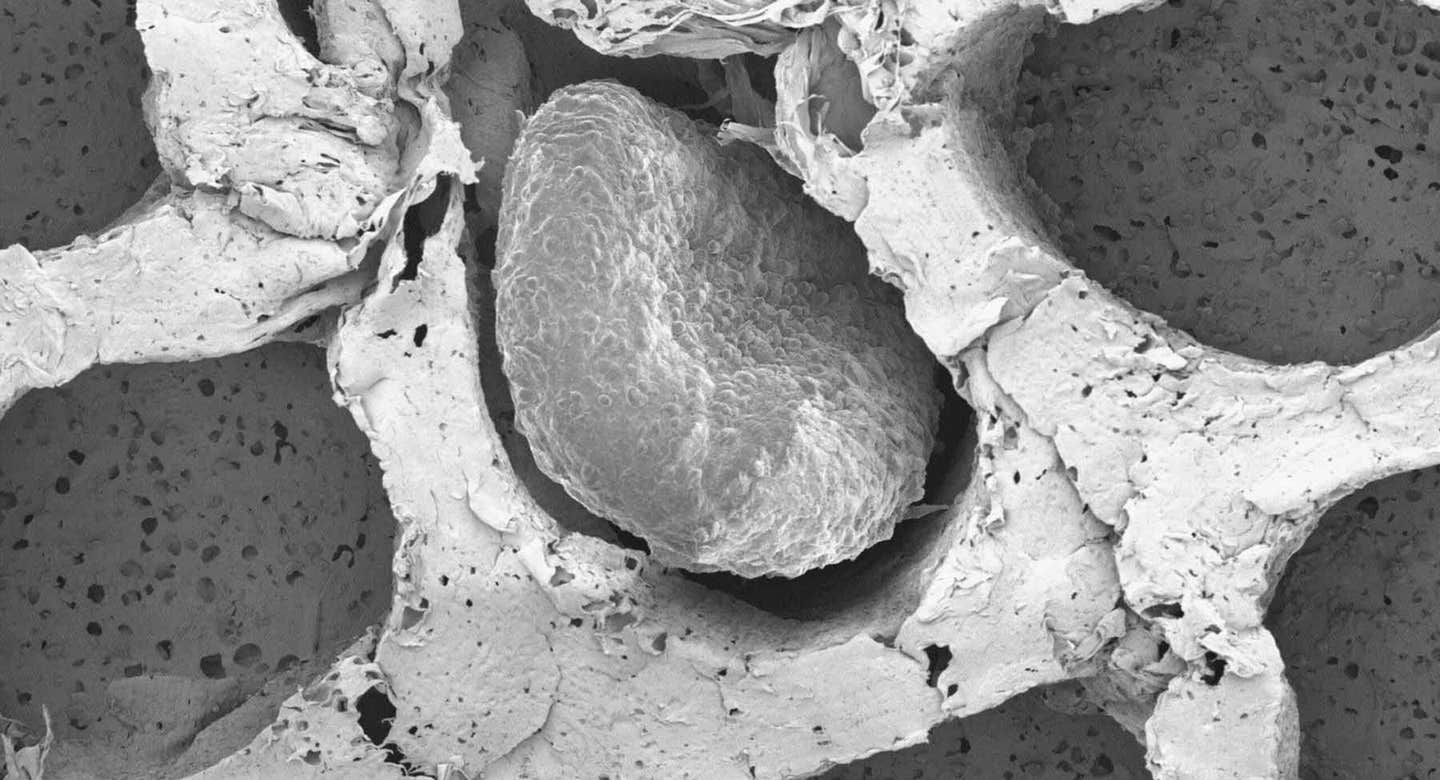
Electron microscopy of fat organoids that were implanted to out-compete tumors. (CREDIT: Desai Lab)
Cancer cells thrive in an environment deprived of oxygen and nutrients, adapting to survive and grow. Unlike normal cells, they rely on a process called aerobic glycolysis, or the Warburg effect, to generate energy. Even in the presence of oxygen, cancer cells preferentially consume glucose, converting it into lactate.
This metabolic reprogramming fuels rapid growth and makes tumors difficult to treat. Researchers have long sought ways to target this unique metabolism, exploring drugs that inhibit glucose and lipid uptake. Now, a new breakthrough may offer a novel approach—using engineered fat cells to starve tumors.
Fat Cells as a Weapon Against Cancer
A research team at UC San Francisco has taken an unexpected approach to cancer therapy by leveraging fat cells. Using CRISPR gene-editing technology, they transformed ordinary white fat cells into energy-burning “beige” fat cells. These modified cells, once implanted near tumors, consume vast amounts of nutrients, depriving cancer cells of their essential fuel.
This strategy takes inspiration from plastic surgery, where fat is routinely removed via liposuction and reintroduced elsewhere in the body. “These fat cells can be easily manipulated in the lab and safely placed back into the body, making them an attractive platform for cellular therapy, including for cancer,” said Nadav Ahituv, PhD, director of the UCSF Institute for Human Genetics and senior author of the paper, which appears in Nature Biotechnology.
A Breakthrough Inspired by Cold Therapy
The idea for this treatment stemmed from earlier studies showing that exposure to cold could suppress cancer in mice. In one case, a patient with non-Hodgkin lymphoma experienced reduced tumor growth when subjected to cold conditions.
Researchers attributed this to the activation of brown fat cells, which consume large amounts of nutrients to generate heat. However, prolonged cold exposure isn’t a practical option for cancer patients, prompting Ahituv and his team to seek an alternative.
Hai Nguyen, PhD, a postdoctoral researcher at UCSF, used CRISPR to activate genes in white fat cells, making them behave like brown fat. The key gene, UCP1, stood out as the most effective. When these engineered fat cells were placed in a petri dish alongside cancer cells, they rapidly consumed the shared nutrients, leaving most of the cancer cells to die.
Related Stories
“In our very first trans-well experiment, very few cancer cells survived. We thought we had messed something up—we were sure it was a mistake,” Ahituv recalled. “So, we repeated it multiple times, and we kept seeing the same effect.”
The modified fat cells effectively starved different types of cancer, including breast, colon, pancreatic, and prostate cancer cells.
Testing the Approach in Living Organisms
To see if the therapy worked in a realistic setting, researchers implanted the engineered fat cells next to tumors in mice. The results were remarkable. The tumors shrank as the fat cells depleted their nutrient supply. Even when placed far from the tumors, the fat cells still managed to outcompete the cancer cells, suppressing tumor growth in genetically predisposed mice.
Further testing involved human breast cancer samples. Jennifer Rosenbluth, MD, PhD, a breast cancer specialist at UCSF, helped the team analyze mastectomy tissue containing both fat and cancer cells. They introduced the engineered fat cells into these samples, observing similar results—beige fat outcompeted the cancer cells for nutrients.
The researchers then took the concept a step further, tailoring fat cells to target specific metabolic dependencies. Some pancreatic cancers, for example, rely on uridine when glucose is unavailable. By programming fat cells to consume uridine, they were able to weaken these tumors significantly. This adaptability suggests that the approach could be customized for different cancer types.
A New Era for Cellular Therapy
Engineered fat cells offer several advantages over traditional therapies. They are easy to obtain, grow well in the lab, and can be genetically modified to suit specific needs. Unlike other cell-based treatments, such as stem cell therapies, fat cells tend to remain in place once implanted, reducing the risk of unintended effects.
“With fat cells, there’s less interaction with the environment, so there’s very little worry of the cells leaking out into the body, where they might cause problems,” Ahituv said.
Beyond cancer, this technology could have other medical applications. Engineered fat cells could be designed to monitor glucose levels and release insulin for diabetes or regulate iron absorption in conditions like hemochromatosis. The possibilities are vast, and researchers believe this method could pave the way for a new class of living cell therapies.
“The sky’s the limit for these fat cells,” Ahituv said.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joseph Shavit
Head Science News Writer | Communicating Innovation & Discovery
Based in Los Angeles, Joseph Shavit is an accomplished science journalist, head science news writer and co-founder at The Brighter Side of News, where he translates cutting-edge discoveries into compelling stories for a broad audience. With a strong background spanning science, business, product management, media leadership, and entrepreneurship, Joseph brings a unique perspective to science communication. His expertise allows him to uncover the intersection of technological advancements and market potential, shedding light on how groundbreaking research evolves into transformative products and industries.