Bioluminescent lanterns shed new light on memory formation and synaptic activity
Discover UCI’s bioluminescent RNA lanterns, a groundbreaking tool enabling real-time imaging of RNA in living organisms, revolutionizing research.
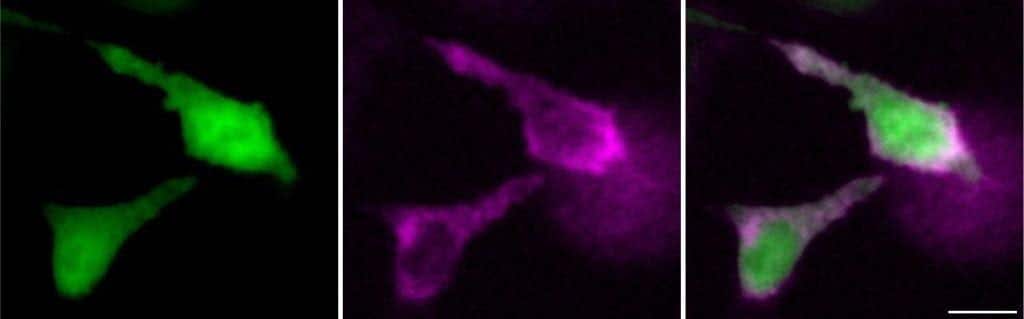
Scientists at UCI have developed bioluminescent RNA lanterns, providing real-time insights into RNA dynamics in living cells and organisms. (CREDIT: UC Irvine)
Bioluminescent RNA lanterns have revolutionized the way researchers track RNA activity within living organisms. In a groundbreaking study, scientists at the University of California, Irvine (UCI) developed a novel platform for imaging RNA in real time, providing unprecedented insights into cellular processes and disease progression.
This innovation, published in Nature Communications, combines advanced luciferase technology with RNA tagging, enabling dynamic visualization of RNA without invasive procedures or external excitation light.
RNA plays a central role in translating genetic information into cellular action. It serves as the intermediary between DNA and proteins, driving essential functions such as growth, adaptation, and memory formation. Until now, tracking RNA’s behavior in living cells has been a significant challenge due to the limitations of existing technologies.
Fluorescent probes, while useful, often require external excitation light, leading to issues like autofluorescence and phototoxicity. These drawbacks hinder the detection of low-abundance targets and limit the ability to observe RNA dynamics in real-time.
The UCI team’s solution leverages the natural luminescence of luciferase enzymes, the same molecules that give fireflies their glow. By engineering a split luciferase system—known as NanoBiT—and fusing it to RNA-binding proteins, the researchers created "RNA lanterns" capable of emitting light upon RNA binding. This approach eliminates the need for external light sources, significantly enhancing sensitivity and accuracy.
The design of the RNA lanterns required meticulous optimization. The team utilized MS2 and PP7 bacteriophage coat proteins (MCP and PCP), which bind specific RNA sequences called aptamers. These aptamers were appended to RNA transcripts, serving as targets for the lantern components. When MCP and PCP bound their respective aptamers, they brought the split luciferase fragments into close proximity, triggering light production.
To enhance the platform’s efficiency, the researchers modeled RNA lantern assembly using ChimeraX software. They ensured that the RNA bait’s structure—a compact and rigid design—minimized steric clashes and maximized luciferase complementation. This precision engineering resulted in a system capable of producing robust luminescence with minimal background noise.
The platform’s versatility was further demonstrated by its ability to adapt to various RNA baits. By modifying the length and orientation of the aptamers, the team optimized photon production. For instance, the M-3-P RNA bait, with its short linker, yielded a 330-fold increase in luminescence compared to controls. This adaptability ensures that RNA lanterns can be tailored to diverse experimental needs.
Biochemical optimization also played a critical role in enhancing the system’s performance. The researchers tested different SmBiT peptide sequences to identify the combination that provided the best dynamic range. SmBiTlow, a peptide with reduced background luminescence, emerged as the optimal choice. By fine-tuning the linkers and orientations of the RNA bait and lantern components, the team achieved high sensitivity and specificity for RNA detection.
Related Stories
The implications of this technology are profound. One of the most promising applications lies in neuroscience, where RNA dynamics are closely linked to memory formation and synaptic activity.
Co-lead author Jennifer Prescher emphasized the potential to observe RNA transport within neurons, a process critical for forming connections at synapses. "Being able to see early events and the transport of RNA directly correlates with memory formation," Prescher explained. This capability could unlock new understanding of how memories are formed and retained, a long-standing goal in neuroscience.
Another significant application is in virology. RNA lanterns can be used to track the propagation of viral RNA within living organisms, providing insights into how viruses infiltrate host cells and evade immune defenses. This real-time imaging capability could inform the development of antiviral therapies and vaccines by revealing vulnerabilities in viral replication processes.
RNA lanterns also hold potential for advancing cancer research. Tumor cells often exhibit unique RNA signatures, and the ability to track these transcripts in real-time could shed light on the molecular mechanisms driving cancer progression. Additionally, the platform could aid in evaluating the effectiveness of targeted therapies by monitoring RNA changes in response to treatment.
Unlike traditional fluorescent probes, RNA lanterns are well-suited for use in live animals. The bioluminescent platform eliminates the need for external light, avoiding issues like autofluorescence and tissue damage. This makes it possible to conduct serial imaging over extended periods, capturing dynamic RNA activity without disrupting the organism’s natural processes.
One key innovation was the use of luciferase enzymes with high photon output and thermal stability. These enzymes enabled the visualization of cellular events at both micro and macro scales. For example, the RNA lanterns successfully detected RNA activity in live mammalian cells and whole organisms, demonstrating their effectiveness in physiologically authentic environments.
Lead author Andrej Lupták highlighted the significance of these advancements: "The first step in understanding cellular changes—whether it’s growth, adaptation, or disease—is understanding RNA dynamics. This tool allows us to visualize RNA like never before."
The platform’s modularity is another strength. Researchers can easily adapt RNA lanterns for specific applications by modifying the RNA bait or lantern components. This flexibility ensures that the technology remains relevant across diverse fields, from basic biology to clinical research.
The development of RNA lanterns underscores the importance of interdisciplinary collaboration. The UCI team brought together experts from pharmaceutical sciences, chemistry, and neurobiology, fostering a synergistic research environment. Students and faculty alike contributed to refining the platform, ensuring its robustness and scalability.
Looking ahead, the researchers aim to expand the RNA lantern toolkit to study more complex RNA behaviors and interactions. This includes exploring multi-scale imaging applications and integrating the platform with other bioluminescent technologies. Additionally, the modular design of the RNA lanterns allows for customization, paving the way for targeted studies in cancer, neurodegenerative diseases, and regenerative medicine.
The potential for RNA lanterns to transform biological research is immense. By providing a window into RNA’s role in health and disease, this innovation sets the stage for breakthroughs in diagnostics, therapeutics, and our fundamental understanding of life.
This innovation could also play a role in environmental science, as researchers explore RNA's function in microbial ecosystems. By tagging RNA in bacteria or other microorganisms, scientists could track their roles in nutrient cycling, pollution degradation, or climate change processes. The insights gained could inform efforts to engineer microorganisms for environmental remediation or biofuel production.
Additionally, the use of RNA lanterns in regenerative medicine could address critical gaps in understanding how RNA influences tissue repair and regeneration. For example, researchers could track RNA dynamics in stem cells as they differentiate into specialized cell types, offering clues on how to optimize treatments for conditions like spinal cord injuries or degenerative diseases.
Finally, the ability to visualize RNA in real-time holds promise for education and public engagement. By making cellular processes visible and comprehensible, RNA lanterns could inspire the next generation of scientists and increase public appreciation for molecular biology.
The research team’s achievements reflect the power of combining innovative technology with collaborative science. As co-author Jennifer Prescher noted, "We had an absolute dream team of students and faculty working together to make this possible. The cross-disciplinary environment at UCI was essential to our success."
As the field of bioluminescent imaging continues to evolve, RNA lanterns are poised to play a central role in unlocking the mysteries of RNA biology. Their ability to provide real-time, noninvasive insights into cellular processes is a testament to the ingenuity and dedication of the scientific community.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joshua Shavit
Science & Technology Writer | AI and Robotics Reporter
Joshua Shavit is a Los Angeles-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a contributor to The Brighter Side of News, he focuses on positive and transformative advancements in AI, technology, physics, engineering, robotics and space science. Joshua is currently working towards a Bachelor of Science in Business Administration at the University of California, Berkeley. He combines his academic background with a talent for storytelling, making complex scientific discoveries engaging and accessible. His work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.



