500 million-year-old fossil reveals evolution of early nervous systems
Discover how ancient worm fossils unveil the evolution of ventral nerve cords in ecdysozoans, shedding light on neural adaptations over 535 million years.
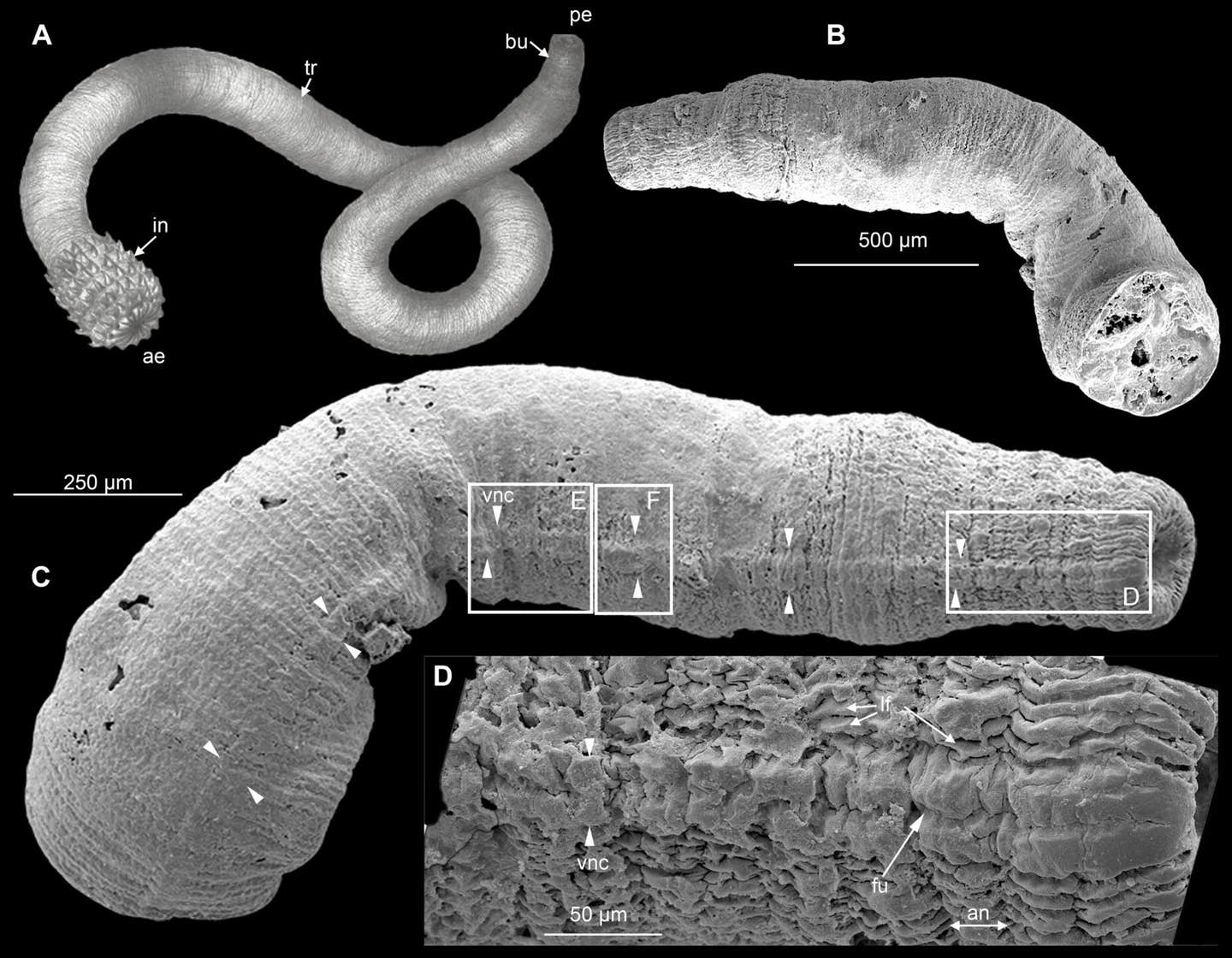
New research on Cambrian fossils reveals how ventral nerve cords evolved in ancient ecdysozoans, offering insights into neural and evolutionary history. (CREDIT: Science)
For over half a billion years, evolution has sculpted the nervous systems of bilaterian animals, equipping them with remarkable capabilities to process information, coordinate movements, and respond to environmental changes.
Among the most intriguing discoveries lies within the ventral nerve cord (VNC), a key component of the central nervous system in ecdysozoans—a diverse group of animals that includes insects, roundworms, and priapulid worms.
Recent research has unveiled new details about the origins and evolution of these structures, shedding light on their emergence during the basal Cambrian period, around 535 million years ago.
A collaborative study involving researchers from Northwest University, Université de Lyon, Queen Mary University of London, Rey Juan Carlos University, and published in the journal, Science, has provided unprecedented insights into the nervous systems of ancient ecdysozoans.
By analyzing fossils from sites like the Kuanchuanpu Formation, Chengjiang Biota, and Burgess Shale, the team uncovered impressions of VNCs in species such as Eopriapulites sphinx, Eokinorhynchus rarus, and Ottoia prolifica.
These fossils, preserved through carbonaceous compression or phosphatization, revealed elongate structures along the ventral side of these early organisms.
Dr. Deng Wang, one of the lead researchers, explained, “These structures closely resemble the ventral nerve cords seen in modern priapulids. They offer a rare glimpse into the architecture of ancient nervous systems, providing clues to the evolutionary trajectory of ecdysozoan lineages.”
The findings suggest that the ancestral condition of scalidophorans, a subgroup of ecdysozoans, featured an unpaired VNC. This structure likely extended along the trunk in an eccentric position, akin to that observed in modern priapulids and nematodes. Such insights illuminate the possible ground patterns of the VNC and its evolutionary significance among early ecdysozoans.
The fossils analyzed were particularly well-preserved due to the unique conditions of their burial environments. The Kuanchuanpu Formation, for example, is known for its phosphatized fossils, allowing for three-dimensional reconstruction of ancient organisms.
The Chengjiang Biota, on the other hand, provides examples of carbonaceous compression fossils that offer a different but equally valuable perspective on soft tissue preservation. These complementary datasets enhance our understanding of the nervous systems of early Cambrian animals and highlight the diversity of preservation modes.
Related Stories
Ecdysozoans are characterized by a ring-like brain and a ventral nerve cord. However, the neural organization varies significantly across subgroups. Priapulids and nematodes possess a single VNC, while kinorhynchs and loriciferans display paired cords, often accompanied by ganglia. These differences raise questions about the evolutionary origins of these features.
Dr. Chema Martin-Durán noted, “The common ancestor of all ecdysozoans likely had a single ventral nerve cord. The paired cords observed in arthropods, kinorhynchs, and loriciferans likely represent independent evolutionary developments, adaptations tied to body segmentation and movement.”
The research team’s phylogenetic analysis supports this hypothesis, suggesting that the single VNC was the ancestral state. Paired nerve cords and ganglia, evident in some lineages, emerged later as derived traits. Such modifications appear to have co-evolved with body segmentation and appendage development, enabling greater motility and environmental adaptability.
Further evidence for this evolutionary pattern comes from comparisons with modern ecdysozoans. Priapulids, often referred to as "penis worms," provide a living example of the ancestral neural condition with their unpaired VNCs.
Kinorhynchs and loriciferans, commonly known as "mud dragons" and "brush heads," respectively, demonstrate more complex neural arrangements that likely arose independently in response to distinct ecological pressures.
The study also emphasizes the role of convergent evolution in shaping nervous system designs. Although the paired VNCs in kinorhynchs and loriciferans resemble those in arthropods, these similarities are not indicative of a shared ancestry. Instead, they reflect parallel adaptations to similar environmental challenges, such as the need for precise locomotion in segmented body plans.
The Cambrian fossils analyzed by the researchers represent pivotal links between ancient and modern ecdysozoans. For instance, Eopriapulites sphinx and Eokinorhynchus rarus from the Fortunian Kuanchuanpu Formation exhibit unpaired VNCs.
Similarly, Xiaoheiqingella peculiaris and Ottoia prolifica, preserved in the Chengjiang Biota and Burgess Shale, retain nervous system structures indicative of their primitive neural architecture.
Dr. Jean Vannier remarked, “These fossils are invaluable for reconstructing the evolutionary relationships within ecdysozoans. They demonstrate that ancient scalidophorans shared neural designs with modern priapulids, highlighting continuity and divergence within the group.”
The study’s findings also shed light on the broader evolutionary history of ecdysozoans. By integrating fossil evidence with phylogenetic analyses, researchers can trace the development of nervous system traits over hundreds of millions of years.
This approach not only clarifies the relationships among modern ecdysozoans but also provides insights into how neural adaptations influenced the diversification of these animals during the Cambrian explosion.
The researchers’ methods included advanced imaging techniques to analyze fossilized nervous systems. High-resolution scanning and three-dimensional modeling allowed them to visualize the fine details of VNC structures. These technologies have revolutionized paleontology, enabling scientists to extract valuable information from even the most challenging fossil specimens.
The emergence of paired nerve cords and ganglia in some ecdysozoans marks a significant evolutionary milestone. These features correlate with body segmentation, a trait seen in kinorhynchs, loriciferans, and panarthropods.
During the Precambrian-Cambrian transition, structural changes in nervous and muscular systems likely facilitated the development of appendages, enabling complex locomotion.
Dr. María Herranz emphasized, “Paired nerve cords allowed for greater coordination of movement, particularly in segmented animals. This advancement likely played a crucial role in the diversification of ecdysozoan lineages during the Cambrian explosion.”
The study underscores the importance of the fossil record in understanding evolutionary processes. Fossils not only provide direct evidence of ancient organisms but also offer a window into the functional and ecological contexts in which these animals lived.
By studying the nervous systems of early ecdysozoans, researchers can infer how these structures influenced behavior, interaction with the environment, and survival strategies.
Moreover, the evolution of nervous systems in ecdysozoans reflects broader trends in animal evolution. The transition from simple to complex neural architectures parallels the development of other key traits, such as segmentation, appendages, and sensory organs.
These changes were likely driven by a combination of genetic innovations and ecological pressures, highlighting the dynamic interplay between biology and environment in shaping evolutionary trajectories.
This groundbreaking research not only advances our understanding of ecdysozoan evolution but also has broader implications for the study of nervous systems in other animal groups. By linking neural structures to evolutionary trends, the study provides a framework for exploring the origins and diversification of complex traits across the animal kingdom.
The findings also underscore the value of interdisciplinary collaboration in scientific research. By combining expertise in paleontology, evolutionary biology, and advanced imaging technologies, the team was able to address fundamental questions about early animal evolution.
Their work highlights the importance of integrating multiple lines of evidence to build a comprehensive picture of life’s history.
Looking ahead, future research could explore how genetic and developmental mechanisms contributed to the evolution of ecdysozoan nervous systems. Advances in genomics and molecular biology may reveal the genetic basis for neural innovations, offering new insights into the evolutionary processes that shaped these animals’ remarkable diversity.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joshua Shavit
Science & Technology Writer | AI and Robotics Reporter
Joshua Shavit is a Los Angeles-based science and technology writer with a passion for exploring the breakthroughs shaping the future. As a contributor to The Brighter Side of News, he focuses on positive and transformative advancements in AI, technology, physics, engineering, robotics and space science. Joshua is currently working towards a Bachelor of Science in Business Administration at the University of California, Berkeley. He combines his academic background with a talent for storytelling, making complex scientific discoveries engaging and accessible. His work highlights the innovators behind the ideas, bringing readers closer to the people driving progress.



