FDA-approved sleeping pill offers new hope in the fight against Alzheimer’s disease
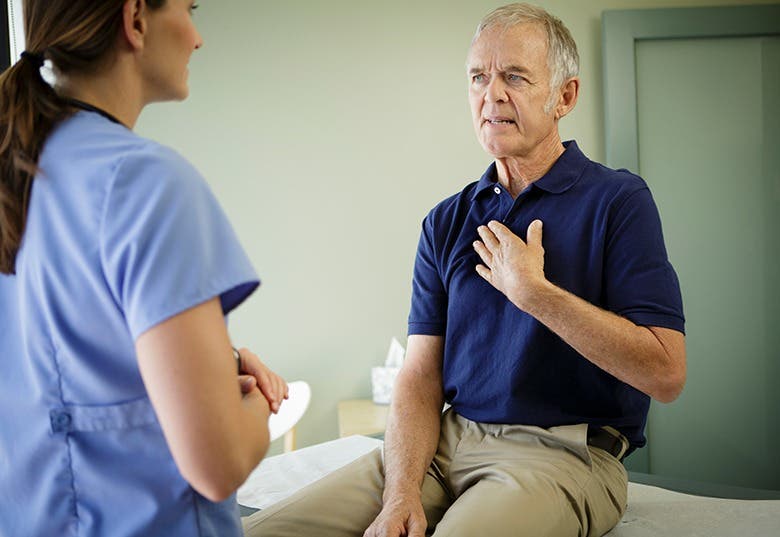
A recent study reveals that a common sleeping pill may reduce harmful Alzheimer’s proteins, offering hope for prevention strategies.

A common sleeping pill may reduce harmful Alzheimer’s proteins, offering hope for prevention strategies. (CREDIT: CC BY-SA 4.0)
Alzheimer’s disease is notorious for robbing individuals of memory and cognitive function. Researchers have struggled for decades to find effective treatments, yet none have successfully halted or reversed the condition.
Now, a study, published in the journal Annals of Neurology, points to an unexpected solution: a medication commonly used to help people sleep.
Scientists recently discovered that a sleeping pill, already FDA-approved to treat insomnia, can temporarily reduce harmful proteins associated with Alzheimer's. The drug, known as suvorexant, targets the protein build-up that characterizes Alzheimer's, potentially slowing its progression.
This discovery adds weight to the growing understanding that sleep is critical in Alzheimer's prevention. Poor sleep can contribute to the disease’s early development, causing harmful proteins to accumulate in the brain. Better sleep might help clear these proteins, offering hope for prevention or delay.
The Sleep-Alzheimer's Connection
Alzheimer’s disease begins silently, often long before noticeable cognitive issues appear. Trouble sleeping can serve as an early warning sign, showing up years before memory loss. Poor sleep quality raises levels of two critical proteins—amyloid-beta and tau—in the brain. These proteins build up into plaques and tangles, eventually causing neurons to die.
Previous research found a direct link between disrupted sleep and higher levels of amyloid-beta and tau. Even one night of poor sleep can cause amyloid-beta to spike, speeding its accumulation. Better sleep quality might flush out these harmful proteins, slowing Alzheimer's disease progression.
Yet, treating sleep issues isn't as straightforward as taking any sleeping pill. Most sleep medications help people fall asleep quickly but may limit the deeper, restorative sleep phases. These phases are crucial to clearing harmful brain proteins. Thus, finding a medication that truly enhances sleep quality is essential.
Related Stories
Suvorexant: More Than Just a Sleep Aid?
In a small but compelling study led by Dr. Brendan Lucey at Washington University School of Medicine, scientists explored how suvorexant affects Alzheimer's-linked proteins. Suvorexant belongs to a new class of sleep medications called dual orexin receptor antagonists. Orexin is a molecule that promotes wakefulness; suvorexant blocks it, promoting deeper sleep.
The study involved 38 healthy adults, ages 45 to 65, without sleep problems or signs of cognitive impairment. Participants spent two nights at a sleep clinic, receiving either suvorexant or a placebo pill. Researchers collected cerebrospinal fluid—a clear liquid surrounding the brain—every two hours through a small tube placed in participants' lower backs. This fluid carries waste away from the brain, including amyloid-beta and tau.
Remarkably, after just one dose of suvorexant, amyloid-beta levels dropped by 10% to 20%, compared to the placebo. A higher dose (20 mg, typically prescribed for insomnia) also lowered a particular form of tau protein, phosphorylated tau, by about 10% to 15%. Phosphorylated tau is crucial because it directly forms harmful tangles that destroy brain cells.
"This is a positive sign," said Dr. Lucey, director of Washington University's Sleep Medicine Center. "If you can reduce tau phosphorylation, potentially there would be less tangle formation and less neuronal death."
Yet, the benefits were temporary. Tau protein levels returned to normal within 24 hours after taking suvorexant. However, repeated nightly doses might keep harmful proteins at consistently lower levels. Dr. Lucey emphasized caution, though: "It would be premature for people who are worried about developing Alzheimer's to interpret it as a reason to start taking suvorexant every night."
Limitations and Future Directions
Despite the encouraging results, researchers urge caution. This initial study was brief, involving only two nights and a small group. It did not assess the long-term impacts of taking suvorexant regularly, nor did it test older adults at higher risk for Alzheimer’s.
Scientists still aren't certain whether lowering amyloid-beta and tau temporarily will prevent Alzheimer's altogether. Previous studies have questioned the idea that reducing these proteins is enough to stop or slow the disease. Many treatments that successfully reduce amyloid-beta have failed to improve memory or cognitive symptoms in patients.
Nonetheless, Dr. Lucey remains hopeful. He believes future studies, lasting months rather than days, might reveal more lasting effects. His team plans to test suvorexant in older adults already showing early amyloid buildup but not yet experiencing cognitive decline.
"We're not quite there yet," he admitted. "I'm hopeful that we will eventually develop drugs that take advantage of the link between sleep and Alzheimer's to prevent cognitive decline."
Should You Consider Sleeping Pills?
While promising, these findings don't mean everyone should reach for suvorexant to protect against Alzheimer's. Long-term use of sleeping pills can lead to dependence, reduced effectiveness, and other health risks. They might even negatively impact sleep quality over time.
Experts recommend instead focusing on good sleep hygiene—maintaining regular sleep schedules, creating restful environments, and addressing underlying issues like sleep apnea. Dr. Lucey stresses the importance of improving sleep naturally, advising individuals concerned about Alzheimer’s to consult a sleep specialist rather than relying solely on medication.
Still, this research highlights the intriguing potential of sleep as a powerful preventive measure against Alzheimer's. If future studies confirm that deep, restful sleep can protect the brain from harmful proteins, sleep-based therapies could become a central approach to preventing cognitive decline.
As Dr. Lucey puts it, better sleep "is a sensible approach to improving general brain health at any age."
Suvorexant: A Brief History in Time
Suvorexant, marketed as Belsomra, is the first dual orexin receptor antagonist (DORA) approved for the treatment of insomnia. Developed by Merck & Co., Inc., the drug's journey began in the early 2000s, aiming to address the significant unmet need for effective insomnia treatments.
The high-throughput screening for orexin receptor antagonists commenced in 2005, leading to the discovery and subsequent approval of suvorexant by the U.S. Food and Drug Administration (FDA) in 2014.
Suvorexant functions by selectively inhibiting the orexin receptors OX₁ and OX₂, which play a crucial role in regulating wakefulness. By blocking these receptors, suvorexant diminishes the wake-promoting influence of orexin neuropeptides, thereby facilitating sleep.
This mechanism is distinct from traditional insomnia medications that typically enhance sleep-promoting signals via gamma-aminobutyric acid (GABA) pathways.
Clinical trials have demonstrated the efficacy of suvorexant in improving both sleep onset and maintenance. In pooled analyses of three-month studies, patients receiving 20/15 mg of suvorexant experienced significant enhancements in total sleep time and reductions in wake after sleep onset compared to placebo.
The treatment was generally well-tolerated, with the most common adverse effect being somnolence, which was typically mild to moderate and occurred early in the treatment course.
Beyond primary insomnia, suvorexant has shown promise in addressing sleep disturbances associated with various conditions.
Note: Always consult your doctor or other qualified healthcare provider with any questions you may have regarding a medical condition, procedure, or treatment, whether it is a prescription medication, over-the-counter drug, vitamin, supplement, or herbal alternative.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News' newsletter.
Joseph Shavit
Head Science News Writer | Communicating Innovation & Discovery
Based in Los Angeles, Joseph Shavit is an accomplished science journalist, head science news writer and co-founder at The Brighter Side of News, where he translates cutting-edge discoveries into compelling stories for a broad audience. With a strong background spanning science, business, product management, media leadership, and entrepreneurship, Joseph brings a unique perspective to science communication. His expertise allows him to uncover the intersection of technological advancements and market potential, shedding light on how groundbreaking research evolves into transformative products and industries.